“Nanu-Nanu!”
Who would have guessed that Mork from Ork would have inspired such an interpretation of the scientific study of skin absorption? While researching the topic, it is easy for the reader to be convinced that skin absorbs whatever it touches—just like the Mork & Mindy character and his habit of drinking liquids by sticking his finger in a glass. While this would certainly make wine tasting parties fascinating to observe, the suggestion that skin absorbs whatever is applied to it is an irresponsible exaggeration of the facts.
Skin is a fascinatingly complicated system, designed to protect against external harms (bacteria, UV radiation, etc,) regulate body heat, and manage nutrient levels and water loss. Many a chemical is frustrated at the inability to penetrate this protective barrier—but some substances can penetrate the skin and absorb into our bodies. Whether these chemicals cause harm depends on the amounts that penetrates and are absorbed, and how the chemical acts once it is inside the body. Does it throw a party and set up shop, or do the bouncers of your body show the chemical the door?
Is That a Horny Layer, or are you Just Happy to See Me?
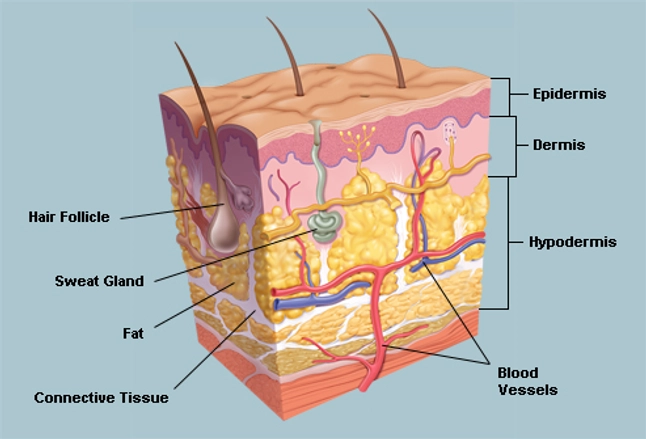
Skin is comprised of three primary regions, the outer epidermis and middle dermis and the lowest area, the hypodermis. The epidermis consists of multiple strata (i.e. layers,) with its superficial the most crucial in prevention of skin penetration. This outermost layer of the epidermis, the stratum corneum (flattened cells, also known as the horny layer,) serves as your body’s primary defense—and it’s dang good at its job.
The stratum corneum, thick with dead skin cells (mostly keratin) and various waxy substances, acts as a wall of protection from external moisture, chemicals, UV radiation and is your foremost guard against the bacterial world. As these dead bits fall of, or exfoliated from the body, the lower layers of skin replenish the surface with additional keratin.
Meet the lower layers! The stratum lucidum is the translucent, second layer of the epidermis in thicker areas, which sits atop the stratum granulosum. The granulosum is responsible for keratin protein production. The stratum spinosum and the lowest layer, the stratum basale, new cells are produced and pushed upwards. Collectively, these layers form the flexible shield against your environment…and that’s the way they all became the Brady Bunch!
Let’s take a closer look (A microscopic glance!) at what substances penetrate skin, and which actually absorb into our bodies!
Penetrating and Absorbing the Facts
Not everything we touch or put on our skin is fully absorbed into our bodies. Otherwise, we’d quite literally be drinking our own bathwater (or swell up like sponges during a swim!) Wait, you say! Doesn’t some water get into our skin to cause it to wrinkle? Remember that dead skin cells fill the stratum corneum—these cells are Jiffy Popped as they soak up the water, which is what causes the “wrinkled” look.
Now, if water were to penetrate the surface layer of skin, we could end up with unpleasant fluid blisters, or as mentioned above never swim in a public pool again. Luckily, this isn’t a risk, and after a short while, the water evaporates and our stratum corneum returns to normal.
The distinction between penetration and absorption is a crucial one where measurement of chemical risk is concerned.
>Skin Penetration represents the amount of a topically chemical that exists between the top layer (stratum corneum) and the bottom layer (stratum basale.) During penetration, the body does not yet absorb the chemical, and it cannot affect the body systems.
>Skin Absorption occurs when the topically applied chemical breaks the skin barrier to reach the bloodstream. Whether this chemical becomes a risk is determined by what occurs after absorption. You body can filter (The bouncers!) out the chemical via bodily fluids, or bio-accumulation (build up) occurs.
Many variables affect the speed (or probability) of penetration and absorption. First, the composition of the chemical to which skin is exposed. The area of skin that is exposed (thinner-skinned areas are more susceptible to penetration and thicker skin is less) and the condition of the skin are all significant factors.
There are a number of scare statistics running about on the internet that state our bodies absorb a tremendous amount of topically applied chemicals. A few interesting ones that I’ve come across:
>Our skin absorbs ____ number of pounds of cosmetics each year
>Our skin absorbs a high percentage of everything we apply to it each day/year/etc.
>A chemical was found in human urine, therefore we are absorbing and slowly marching towards oblivion by said chemical
>If skin didn’t absorb everything we apply to it, then why does a medication patch delivery work so well?
Such statements are exaggerations, distortions, or a little of both where skin penetration and absorption are concerned. The composition of the chemical exposed to skin determines its possibility of entering the skin—primarily the molecule size and solubility of the chemical.
For the purpose of this paper, we’ll limit our scope to chemicals in skin care and cosmetics (you’re welcome!)
The design of cosmetic and skin care formulas is to benefit the outer layer of skin— absorption into the body would waste the effects of these products. Antioxidants in skin care won’t do their job if they don’t stay in the layers of skin—it is challenging enough to develop a formula that enables an ingredient to penetrate the surface layer! The majority of cosmetics are not soluble in skin (i.e. lipid, or fat-soluble) and are too large in molecule to fit through the stratum corneum. Precisely because of these qualities, some skin care formulas require specially developed “penetration enhancers” to deliver ingredients like vitamin C or retinol.
This includes transdermal medication patches! These types of medicine require formulation specifically for this purpose, requiring chemical engineering to create a molecule that is soluble in skin, and small enough to penetrate and absorb into the body.
Much controversy has arisen over the ingredients in skin care products that inadvertently absorb into our body and the possible risk to our health. Does absorption equal harm?
Everything is a Risk, but Not Necessarily a Harm
Absorption into the body doesn’t equate to bodily harm. What happens after a chemical is absorbed makes the distinction! Our bodies design will filter out molecules and water, disposing of what doesn’t belong by excretion via bodily fluids. Safety testing for the effect of ingredients that absorb into our bodies:
>Evaluates how (and how long) we are exposed (topical or oral application, etc)
>Composition of the ingredient, (molecule size, solubility, etc)
>How the ingredient behaves after absorption
These are crucial points for research, as a study of how an ingredient reacted when fed to a test subject is somewhat relevant in understanding what happens when you drink your body lotion—but not as relevant in studies that demonstrate topical application. This is a very, very important point to consider when reading the latest scare report on cosmetics and skin care. Often, such reports cite research that fed large quantities of a pure substance to arrive at their conclusions—hardly honest use of a reference!
Determining the safety of a chemical in skin absorption is about risk assessment. The toxicity of an ingredient is in the amount absorbed and accumulated, or “the dose makes the poison.” The nutrients and substances we depend on for our health can kill us in a large enough amount. When a chemical penetrates our skin and is absorbed into our bodies, is may be converted into another chemical form, metabolized or accumulate.
At the dose in which a chemical becomes harmful (toxic) is the threshold, less than this amount is safe, and more becomes a danger. Our body is designed to break down chemicals into other forms that are easily excreted via fluids. The threshold is the over/under amount of our bodies ability to process a chemical and still keep the body healthy.
Considering this, when you read about the latest scare over a chemical detected in urine, remember what we’ve just discussed! A chemical eliminated from the system isn’t an indicator of threat to health, but your body working as intended and filtering the substance out. The faster this process occurs, the less (if any) impact on your health.
Flushing out the Truth
It’s far too easy to present a frightening statistic in a believable manner, which is why it’s important to question the motives of a flashy headline that proclaims death by lipstick/body lotion/etc. It’s also easy to present distorted research on skin penetration and absorption—as it can be complicated to research to find the truth. The facts are that very little is capable of penetrating skin, and even less is absorbed into our body. So the next time you read a headline, judge for yourself what’s true by asking questions and consider the source!
Who would have guessed that Mork from Ork would have inspired such an interpretation of the scientific study of skin absorption? While researching the topic, it is easy for the reader to be convinced that skin absorbs whatever it touches—just like the Mork & Mindy character and his habit of drinking liquids by sticking his finger in a glass. While this would certainly make wine tasting parties fascinating to observe, the suggestion that skin absorbs whatever is applied to it is an irresponsible exaggeration of the facts.

Skin is a fascinatingly complicated system, designed to protect against external harms (bacteria, UV radiation, etc,) regulate body heat, and manage nutrient levels and water loss. Many a chemical is frustrated at the inability to penetrate this protective barrier—but some substances can penetrate the skin and absorb into our bodies. Whether these chemicals cause harm depends on the amounts that penetrates and are absorbed, and how the chemical acts once it is inside the body. Does it throw a party and set up shop, or do the bouncers of your body show the chemical the door?
Is That a Horny Layer, or are you Just Happy to See Me?
Skin is comprised of three primary regions, the outer epidermis and middle dermis and the lowest area, the hypodermis. The epidermis consists of multiple strata (i.e. layers,) with its superficial the most crucial in prevention of skin penetration. This outermost layer of the epidermis, the stratum corneum (flattened cells, also known as the horny layer,) serves as your body’s primary defense—and it’s dang good at its job.
The stratum corneum, thick with dead skin cells (mostly keratin) and various waxy substances, acts as a wall of protection from external moisture, chemicals, UV radiation and is your foremost guard against the bacterial world. As these dead bits fall of, or exfoliated from the body, the lower layers of skin replenish the surface with additional keratin.
Meet the lower layers! The stratum lucidum is the translucent, second layer of the epidermis in thicker areas, which sits atop the stratum granulosum. The granulosum is responsible for keratin protein production. The stratum spinosum and the lowest layer, the stratum basale, new cells are produced and pushed upwards. Collectively, these layers form the flexible shield against your environment…and that’s the way they all became the Brady Bunch!
Let’s take a closer look (A microscopic glance!) at what substances penetrate skin, and which actually absorb into our bodies!
Penetrating and Absorbing the Facts
Not everything we touch or put on our skin is fully absorbed into our bodies. Otherwise, we’d quite literally be drinking our own bathwater (or swell up like sponges during a swim!) Wait, you say! Doesn’t some water get into our skin to cause it to wrinkle? Remember that dead skin cells fill the stratum corneum—these cells are Jiffy Popped as they soak up the water, which is what causes the “wrinkled” look.
Now, if water were to penetrate the surface layer of skin, we could end up with unpleasant fluid blisters, or as mentioned above never swim in a public pool again. Luckily, this isn’t a risk, and after a short while, the water evaporates and our stratum corneum returns to normal.
The distinction between penetration and absorption is a crucial one where measurement of chemical risk is concerned.
>Skin Penetration represents the amount of a topically chemical that exists between the top layer (stratum corneum) and the bottom layer (stratum basale.) During penetration, the body does not yet absorb the chemical, and it cannot affect the body systems.
>Skin Absorption occurs when the topically applied chemical breaks the skin barrier to reach the bloodstream. Whether this chemical becomes a risk is determined by what occurs after absorption. You body can filter (The bouncers!) out the chemical via bodily fluids, or bio-accumulation (build up) occurs.
Many variables affect the speed (or probability) of penetration and absorption. First, the composition of the chemical to which skin is exposed. The area of skin that is exposed (thinner-skinned areas are more susceptible to penetration and thicker skin is less) and the condition of the skin are all significant factors.
There are a number of scare statistics running about on the internet that state our bodies absorb a tremendous amount of topically applied chemicals. A few interesting ones that I’ve come across:
>Our skin absorbs ____ number of pounds of cosmetics each year
>Our skin absorbs a high percentage of everything we apply to it each day/year/etc.
>A chemical was found in human urine, therefore we are absorbing and slowly marching towards oblivion by said chemical
>If skin didn’t absorb everything we apply to it, then why does a medication patch delivery work so well?
Such statements are exaggerations, distortions, or a little of both where skin penetration and absorption are concerned. The composition of the chemical exposed to skin determines its possibility of entering the skin—primarily the molecule size and solubility of the chemical.
For the purpose of this paper, we’ll limit our scope to chemicals in skin care and cosmetics (you’re welcome!)
The design of cosmetic and skin care formulas is to benefit the outer layer of skin— absorption into the body would waste the effects of these products. Antioxidants in skin care won’t do their job if they don’t stay in the layers of skin—it is challenging enough to develop a formula that enables an ingredient to penetrate the surface layer! The majority of cosmetics are not soluble in skin (i.e. lipid, or fat-soluble) and are too large in molecule to fit through the stratum corneum. Precisely because of these qualities, some skin care formulas require specially developed “penetration enhancers” to deliver ingredients like vitamin C or retinol.
This includes transdermal medication patches! These types of medicine require formulation specifically for this purpose, requiring chemical engineering to create a molecule that is soluble in skin, and small enough to penetrate and absorb into the body.
Much controversy has arisen over the ingredients in skin care products that inadvertently absorb into our body and the possible risk to our health. Does absorption equal harm?
Everything is a Risk, but Not Necessarily a Harm
Absorption into the body doesn’t equate to bodily harm. What happens after a chemical is absorbed makes the distinction! Our bodies design will filter out molecules and water, disposing of what doesn’t belong by excretion via bodily fluids. Safety testing for the effect of ingredients that absorb into our bodies:
>Evaluates how (and how long) we are exposed (topical or oral application, etc)
>Composition of the ingredient, (molecule size, solubility, etc)
>How the ingredient behaves after absorption
These are crucial points for research, as a study of how an ingredient reacted when fed to a test subject is somewhat relevant in understanding what happens when you drink your body lotion—but not as relevant in studies that demonstrate topical application. This is a very, very important point to consider when reading the latest scare report on cosmetics and skin care. Often, such reports cite research that fed large quantities of a pure substance to arrive at their conclusions—hardly honest use of a reference!
Determining the safety of a chemical in skin absorption is about risk assessment. The toxicity of an ingredient is in the amount absorbed and accumulated, or “the dose makes the poison.” The nutrients and substances we depend on for our health can kill us in a large enough amount. When a chemical penetrates our skin and is absorbed into our bodies, is may be converted into another chemical form, metabolized or accumulate.
At the dose in which a chemical becomes harmful (toxic) is the threshold, less than this amount is safe, and more becomes a danger. Our body is designed to break down chemicals into other forms that are easily excreted via fluids. The threshold is the over/under amount of our bodies ability to process a chemical and still keep the body healthy.
Considering this, when you read about the latest scare over a chemical detected in urine, remember what we’ve just discussed! A chemical eliminated from the system isn’t an indicator of threat to health, but your body working as intended and filtering the substance out. The faster this process occurs, the less (if any) impact on your health.
Flushing out the Truth
It’s far too easy to present a frightening statistic in a believable manner, which is why it’s important to question the motives of a flashy headline that proclaims death by lipstick/body lotion/etc. It’s also easy to present distorted research on skin penetration and absorption—as it can be complicated to research to find the truth. The facts are that very little is capable of penetrating skin, and even less is absorbed into our body. So the next time you read a headline, judge for yourself what’s true by asking questions and consider the source!

No comments:
Post a Comment