On the existence of two states in liquid water:
impact on biological and nanoscopic systems
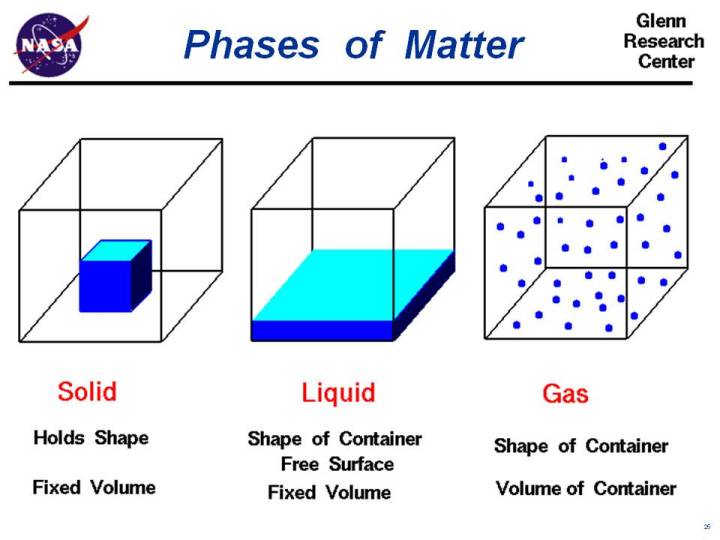
All matter is made from atoms. Every substance (oxygen, lead, silver, neon ...) has a unique number of protons, neutrons, and electrons. Oxygen, for example, has 8 protons, 8 neutrons, and 8 electrons. Hydrogen has 1 proton and 1 electron. Individual atoms can combine with other atoms to form molecules. Water molecules contain two atoms of hydrogen H and one atom of oxygen O and is chemically called H2O. Oxygen and nitrogen are the major components of air and occur in nature as diatomic (two atom) molecules. Regardless of the type of molecule, matter normally exists as either a solid, a liquid, or a gas. We call this property of matter the phase of the matter. The three normal phases of matter have unique characteristics which are listed on the slide.
Solid
In the solid phase the molecules are closely bound to one another by molecular forces. A solid holds its shape and the volume of a solid is fixed by the shape of the solid.
Liquid
In the liquid phase the molecular forces are weaker than in a solid. A liquid will take the shape of its container with a free surface in a gravitational field. In micro-gravity, a liquid forms a ball inside a free surface. Regardless of gravity, a liquid has a fixed volume.
Gas
In the gas phase the molecular forces are very weak. A gas fills its container, taking both the shape and the volume of the container.
Fluids (Liquids and Gases)
Liquids and gases are called fluids because they can be made to flow, or move. In any fluid, the molecules themselves are in constant, random motion, colliding with each other and with the walls of any container. The motion of fluids and the reaction to external forces are described by the Navier-Stokes Equations, which express a conservation of mass, momentum, and energy. The motion of solids and the reaction to external forces are described by Newton's Laws of Motion.
Any substance can occur in any phase. Under standard atmospheric conditions, water exists as a liquid. But if we lower the temperature below 0 degrees Celsius, or 32 degrees Fahrenheit, water changes its phase into a solid called ice. Similarly, if we heat a volume of water above 100 degrees Celsius, or 212 degrees Fahrenheit, water changes its phase into a gas called water vapor. Changes in the phase of matter are physical changes, not chemical changes. A molecule of water vapor has the same chemical composition, H2O, as a molecule of liquid water or a molecule of ice.
When studying gases , we can investigate the motions and interactions of individual molecules, or we can investigate the large scale action of the gas as a whole. Scientists refer to the large scale motion of the gas as the macro scale and the individual molecular motions as the micro scale. Some phenomenon are easier to understand and explain based on the macro scale, while other phenomenon are more easily explained on the micro scale. Macro scale investigations are based on things that we can easily observe and measure. But micro scale investigations are based on rather simple theories because we cannot actually observe an individual gas molecule in motion. Macro scale and micro scale investigations are just two views of the same thing.
Plasma - the "fourth phase"
The three normal phases of matter listed on the slide have been known for many years and studied in physics and chemistry classes. In recent times, we have begun to study matter at the very high temperatures and pressures which typically occur on the Sun, or during re-entry from space. Under these conditions, the atoms themselves begin to break down; electrons are stripped from their orbit around the nucleus leaving a positively charged ion behind. The resulting mixture of neutral atoms, free electrons, and charged ions is called a plasma. A plasma has some unique qualities that causes scientists to label it a "fourth phase" of matter. A plasma is a fluid, like a liquid or gas, but because of the charged particles present in a plasma, it responds to and generates electro-magnetic forces. There are fluid dynamic equations, called the Boltzman equations, which include the electro-magnetic forces with the normal fluid forces of the Navier-Stokes equations. NASA is currently doing research into the use of plasmas for an ion propulsion system.
States of matter, also called “phases”, are a key concept in the study of systems made from atoms and molecules. Roughly speaking, a system formed from many molecules can be arranged in a certain number of configurations depending on its total energy. At higher temperatures (and therefore higher energies), the molecules have more possible configurations and so are more disorganised and can move about relatively freely (the gas phase). At lower temperatures, the molecules have a more limited number of configurations and so form a more ordered phase (a liquid). If the temperature goes down further, they arrange themselves in a very specific configuration, producing a solid.
This picture is common for relatively simple molecules such as carbon dioxide or methane, which have three clear, different states (liquid, solid and gas). But for more complex molecules, there is a larger number of possible configurations and this gives rise to more phases. A beautiful illustration of this is the rich behaviour of liquid crystals, which are formed by complex organic molecules and can flow like liquids, but still have a solid-like crystalline structure
Because the phase of a substance is determined by how its molecules are configured, many physical properties of that substance will change abruptly as it goes from one state to another. In the recent paper, the researchers measured several telltale physical properties of water at temperatures between 0℃ and 100℃ under normal atmospheric conditions (meaning the water was a liquid). Surprisingly, they found a kink in properties such as the water’s surface tension and its refractive index (a measure of how light travels through it) at around 50℃.
Special structure
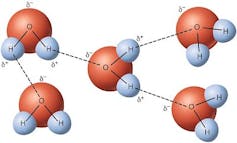
Hydrogen bonds.⇧ Wikimedia Commons, CC BY-SA
How can this be? The structure of a water molecule, H₂O, is very interesting and can be pictured like a sort of arrow tip, with the two hydrogen atoms flanking the oxygen atom at the top. The electrons in the molecule tend to be distributed in a rather asymmetric way, making the oxygen side negatively charged relative to the hydrogen side. This simple structural feature leads to a kind of interaction between water molecules known as hydrogen bonding, in which the opposite charges attract each other.
This gives water properties that, in many cases, break the trends observed for other simple liquids. For example, unlike most other substances, a fixed mass of water takes up more room as a solid (ice) than as a (liquid) because of the way it molecules form a specific regular structure. Another example is the surface tension of liquid water, which is roughly twice that of other non-polar, simpler, liquids.
Water is simple enough, but not too simple. This means that one possibility for explaining the apparent extra phase of water is that it behaves a little bit like a liquid crystal. The hydrogen bonds between molecules keep some order at low temperatures, but eventually could take a second, less-ordered liquid phase at higher temperatures. This could explain the kinks observed by the researchers in their data.
If confirmed, the authors’ findings could have many applications. For example, if changes in the environment (such as temperature) cause changes in a substance’s physical properties, then this can potentially be used for sensing applications. Perhaps more fundamentally, biological systems are mostly made of water. How biological molecules (such as proteins) interact with each other likely depends on the specific manner in which water molecules arrange to form a liquid phase. Understanding how water molecules arrange themselves on average at different temperatures could shed light on the workings of how they interact in biological systems.
The discovery is an exciting opportunity for theorists and experimentalists, and a beautiful example of how even the most familiar substance still has secrets hiding within. The researchers were surprised to find a number of physical properties of water change their behaviour between 50℃ and 60℃. This sign of a potential change to a second liquid state could spark a heated discussion in the scientific community. And, if confirmed, it could have implications for a range of fields, including nanotechnology and biology.
Int. J. Nanotechnol., Vol. 13, Nos. 8/9, 2016. Page 667
On the existence of two states in liquid water:
impact on biological and nanoscopic systems
L.M. Maestro
Clarendon Laboratory,
Department of Physics,
University of Oxford,
Parks Road, Oxford OX1 3PU, UK
Email: laura.martinezmaestro@physics.ox.ac.uk
M.I. Marqués*
Departamento de Física de Materiales,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain.
and
Condensed Matter Physics Center (IFIMAC)
and Instituto Nicolás Cabrera,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain.
Email: manuel.marques@uam.es
*Corresponding author
E. Camarillo
Institute of Physics,
UNAM,
Mexico DF, 04510, México
Email: cgarcia@fisica.unam.mx
D. Jaque and J. García Solé
Departamento de Física de Materiales,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain
Email: daniel.jaque@uam.es
Email: jose.garcia_sole@uam.es
J.A. Gonzalo
Departamento de Física de Materiales,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain
Page 668 L.M. Maestro et al.
and
Escuela Politécnica,
Universidad San Pablo-CEU,
Madrid, 28003, Spain
Email: julio.gonzalo@uam.es
F. Jaque
Departamento de Física de Materiales,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain
Email: francisco.jaque@uam.es
Juan C. del Valle
Departamento de Química Física Aplicada,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain
Email: juan.valle@uam.es
F. Mallamace
Dipartimento di Fisica,
Università di Messina
and Consiglio Nazionale delle Ricerche – Istituto per i Processi
Chimico-Fisici (CNR-IPCF),
Messina, 98158, Italy
Email: francesco.mallamace@unime.it
H.E. Stanley
Center for Polymer Studies, and Department of Physics,
Boston University,
Boston, MA 02215, USA
Email: hes@bu.edu
Abstract: This work reviews several properties of
liquid water, including the dielectric constant and
the proton-spin lattice relaxation, and draws
attention to a bi-linear behaviour defining a
crossover in the temperature range 50 ± 10°C
between two possible states in liquid water. The
existence of these two states in liquid water plays
an important role in nano-metric and biological
systems. For example, the optical properties of
metallic (gold and silver) nano particles dispersed
in water, used as nano-probes, and the emission
properties of CdTe quantum dots (QDs), used for
fluorescence bioimaging and tumour targeting,
show a singular behaviour in this temperature
range. In addition, the structural changes in liquid
water may be associated with the behaviour of biological macromolecules in aqueous solutions
and in particular with protein denaturation.
On the existence of two states in liquid water Page 669
Keywords: water; nanotechnology;
biotechnology; crossover temperature; proteins.
Reference to this paper should be made as
follows: Maestro, L.M.,Marqués, M.I., Camarillo,
E., Jaque, D., García Solé, J., Gonzalo, J.A., Jaque,
F., del Valle, J.C., Mallamace, F. and Stanley, H.E.
(2016) ‘On the existence of two states in liquid
water: impact on biological and nanoscopic
systems’, Int. J. Nanotechnol., Vol. 13, Nos. 8/9,
pp.667–677.
Biographical notes: Laura Martinez Maestro started collaborating with PhD before Daniel Jaque obtaining a Bachelor’s degree in Physics from the Universidad Autónoma de Madrid, Madrid, Spain, in 2009. Her undergraduate research was focused in confocal microscopy to study the behaviour of laser written waveguides in LiNb. She obtained a Masters degree in Photonics from Universidad Autónoma de Madrid in 2010 and defended her PhD thesis, entitle, “Quantum dots as luminescent nano-thermometers: controlled plasmonic hyperthermia” which was awarded with the ‘cum laude’ remark in the same university five years later. In September 2015, she joined Herz and Johnston groups at the Condensed Matter Physics Department in the University of
Oxford as a Postdoctoral Research Assistant to study III–V semiconductor nano-wires.
Manuel I. Marqués obtained his BA in Physics at Universidad Complutense de Madrid in 1995 and was awarded with an extraordinary PhD prize in Physics at Universidad Autónoma de Madrid in 2000 under the supervision of Professor Julio A. Gonzalo. He is a Fullbright Fellow at Boston University from 2001 to 2003 where he performed a postdoctoral research in the group of Professor Gene Stanley. In 2003 he was awarded with a Ramón y Cajal appointment at the Universidad Autónoma de Madrid. He is now an Associate Professor in the Material Physics Department. His research interests, mainly focused on phase transitions and light matter interactions, have been awarded with a National Prize of the Cuban Academy of Sciences (2015).
Enrique Camarillo received the BS, MS and a PhD in Physics from the University National Autonomous of México, UNAM, in 1971, 1985 and 1991, respectively. He was a Postdoctoral Research Fellow in the University Autonomous of Madrid, UAM. Currently, he is working at the Institute of Physics, UNAM, on Optical Properties of Solids.
Daniel Jaque is a Professor at Universidad Autónoma de Madrid. In 2006, he was awarded with the “Young Researcher Award” by the European Association for the Study of Rare Earths and Actanides. That same year, he moved his research interest towards the micro/nano structuration of optical materials by ultrafast laser inscription covering not only practical aspects but also fundamental ones. Indeed, he has been a pioneer in the application of confocal fluorescence imaging techniques for the understanding of the
light-matter interaction at the femtosecond time scale. The know-how acquired on confocal fluorescence imaging techniques has allowed him to face new research areas such as fluorescence imaging of living cancer cells and fluids. In 2009, he was an invited Visiting Professor in Heriot Watt University and in Swinburne University of Technology.
José García Solé is a Professor of Applied Physics at Universidad Autónoma de Madrid. He obtained an Invited Professor position at the University of Lyon (France) (1993) and was awarded by a Full Professor position (Cátedra “Elena Aizen de Moshinsky”) at the Universidad Nacional Autónoma de México (1994). Recently, he has been also awarded with a special recognition
Page 670 L.M. Maestro et al.
by University of Sonora (Hermosillo, México) (November, 2012). His research interest lies on the study of novel optical materials, multifunctional solid state lasers and laser physics. During the last years his research activity has been mostly focused to fluorescence bioimaging and to study new inorganic optical nanocrystals for biomedical applications. He is been co-author of almost 300 peer review papers in international journals and seven books. Among them it does stand out the book “An Introduction to the Optical Spectroscopy of Inorganic Solids” John Wiley & Sons (2005).
Julio A. Gonzalo has a PhD in Physics (1962) from the Universidad
Complutense, Madrid. He did research and teaching at Salamanca (Spain), Mayagüez (Puerto Rico), Rio Piedras (Puerto Rico), Barcelona (Spain) and UAM Madrid (Spain). He worked as Research Collaborator at Brookhaven National Laboratory (US-AEC) and was División Head and Sr. Scientist at PRNC (US-AEC). He has been author or co-author of more than a dozen
scientific books, including ‘Cosmic Paradoxes’ (World Scientific: Singapore, 2012) of which a 2nd edition will appear in 2016.
Francisco Jaque received the BS and the PhD in Physics from the Universidad Complutense of Madrid, Madrid, Spain, in 1970 and 1975, respectively. He was a Postdoctoral Research Fellow in the University of Orsay (France), Sussex (UK), Parma (Italy) Strathclyde, Scotland (UK) and Instituto de Física of Mexico (Mexico) working in Radiation Damage and Optical Proprieties
of Solids. Since 1982, he is a Full Professor at the Department of Materials Sciences, Universidad Autónoma of Madrid. During the period 2012–2016, he was the University Ombudsman.
Juan Carlos del Valle is an Associate Professor of Applied Physical Chemistry at the University “Autónoma de Madrid”. He obtained his PhD in 1994 at the University mentioned above under supervision of Professor Javier Catalán, and did postdoctoral studies at Florida State University collaborating with Professor
Michael Kasha. His research focuses on the solvatochromism of organic molecules, the excited state proton transfer reactions of molecular systems possessing intra- and inter-molecular hydrogen bonds and their applications as molecular probes in biological systems or as dye-lasers. His current research includes solvatochromism of molecular probes in water and in water/urea
solvent mixtures.
Francesco Mallamace received his BE from the Department of Physics at Messina University in 1973. In 1979, he became Professor of Physics. He started his scientific career at Rome La Sapienza by working on laser experiments related with the theory of coherence of light. After that, he worked on the physics of complex liquids and systems by studying their thermodynamic properties from the stable to the supercooled regime by using
several different experimental approaches, such as scattering (light and neutron), viscoelasticity, sound propagation, and nuclear magnetic resonance. In all of these studies, his approach is based on the use of the proper models of statistical physics. His current research interests include water and dynamical properties of glass forming materials (molecular or polymeric) on approaching
the arrested-glassy state.
H. Eugene Stanley works in collaboration with students and colleagues attempting to understand puzzles of interdisciplinary science. He has been elected to the U.S. National Academy of Sciences (NAS) and received the 2004 IUPAP Boltzmann Medal. His main current focus understands the anomalous behaviour of liquid water in bulk, nanoconfined, and biological environments.
He has also worked on a range of other topics in complex systems, such as
On the existence of two states in liquid water. Page 671
quantifying correlations among the constituents of the Alzheimer brain and quantifying fluctuations in noncoding and coding DNA sequences, and interbeat intervals of the healthy and diseased heart. His publications, all available online at http://polymer.bu.edu/hes/ have received more than 80,000 ISI Web of Science citations and more than 120,000 citations in Google Scholar. His ISI Hirsch index is currently h = 128. He has served as thesis advisor to 114 PhD candidates at MIT and Boston University.
1 Introduction
impact on biological and nanoscopic systems

All matter is made from atoms. Every substance (oxygen, lead, silver, neon ...) has a unique number of protons, neutrons, and electrons. Oxygen, for example, has 8 protons, 8 neutrons, and 8 electrons. Hydrogen has 1 proton and 1 electron. Individual atoms can combine with other atoms to form molecules. Water molecules contain two atoms of hydrogen H and one atom of oxygen O and is chemically called H2O. Oxygen and nitrogen are the major components of air and occur in nature as diatomic (two atom) molecules. Regardless of the type of molecule, matter normally exists as either a solid, a liquid, or a gas. We call this property of matter the phase of the matter. The three normal phases of matter have unique characteristics which are listed on the slide.
Solid
In the solid phase the molecules are closely bound to one another by molecular forces. A solid holds its shape and the volume of a solid is fixed by the shape of the solid.
Liquid
In the liquid phase the molecular forces are weaker than in a solid. A liquid will take the shape of its container with a free surface in a gravitational field. In micro-gravity, a liquid forms a ball inside a free surface. Regardless of gravity, a liquid has a fixed volume.
Gas
In the gas phase the molecular forces are very weak. A gas fills its container, taking both the shape and the volume of the container.
Fluids (Liquids and Gases)
Liquids and gases are called fluids because they can be made to flow, or move. In any fluid, the molecules themselves are in constant, random motion, colliding with each other and with the walls of any container. The motion of fluids and the reaction to external forces are described by the Navier-Stokes Equations, which express a conservation of mass, momentum, and energy. The motion of solids and the reaction to external forces are described by Newton's Laws of Motion.
Any substance can occur in any phase. Under standard atmospheric conditions, water exists as a liquid. But if we lower the temperature below 0 degrees Celsius, or 32 degrees Fahrenheit, water changes its phase into a solid called ice. Similarly, if we heat a volume of water above 100 degrees Celsius, or 212 degrees Fahrenheit, water changes its phase into a gas called water vapor. Changes in the phase of matter are physical changes, not chemical changes. A molecule of water vapor has the same chemical composition, H2O, as a molecule of liquid water or a molecule of ice.
When studying gases , we can investigate the motions and interactions of individual molecules, or we can investigate the large scale action of the gas as a whole. Scientists refer to the large scale motion of the gas as the macro scale and the individual molecular motions as the micro scale. Some phenomenon are easier to understand and explain based on the macro scale, while other phenomenon are more easily explained on the micro scale. Macro scale investigations are based on things that we can easily observe and measure. But micro scale investigations are based on rather simple theories because we cannot actually observe an individual gas molecule in motion. Macro scale and micro scale investigations are just two views of the same thing.
Plasma - the "fourth phase"
The three normal phases of matter listed on the slide have been known for many years and studied in physics and chemistry classes. In recent times, we have begun to study matter at the very high temperatures and pressures which typically occur on the Sun, or during re-entry from space. Under these conditions, the atoms themselves begin to break down; electrons are stripped from their orbit around the nucleus leaving a positively charged ion behind. The resulting mixture of neutral atoms, free electrons, and charged ions is called a plasma. A plasma has some unique qualities that causes scientists to label it a "fourth phase" of matter. A plasma is a fluid, like a liquid or gas, but because of the charged particles present in a plasma, it responds to and generates electro-magnetic forces. There are fluid dynamic equations, called the Boltzman equations, which include the electro-magnetic forces with the normal fluid forces of the Navier-Stokes equations. NASA is currently doing research into the use of plasmas for an ion propulsion system.
States of matter, also called “phases”, are a key concept in the study of systems made from atoms and molecules. Roughly speaking, a system formed from many molecules can be arranged in a certain number of configurations depending on its total energy. At higher temperatures (and therefore higher energies), the molecules have more possible configurations and so are more disorganised and can move about relatively freely (the gas phase). At lower temperatures, the molecules have a more limited number of configurations and so form a more ordered phase (a liquid). If the temperature goes down further, they arrange themselves in a very specific configuration, producing a solid.
This picture is common for relatively simple molecules such as carbon dioxide or methane, which have three clear, different states (liquid, solid and gas). But for more complex molecules, there is a larger number of possible configurations and this gives rise to more phases. A beautiful illustration of this is the rich behaviour of liquid crystals, which are formed by complex organic molecules and can flow like liquids, but still have a solid-like crystalline structure
Because the phase of a substance is determined by how its molecules are configured, many physical properties of that substance will change abruptly as it goes from one state to another. In the recent paper, the researchers measured several telltale physical properties of water at temperatures between 0℃ and 100℃ under normal atmospheric conditions (meaning the water was a liquid). Surprisingly, they found a kink in properties such as the water’s surface tension and its refractive index (a measure of how light travels through it) at around 50℃.
Special structure

Hydrogen bonds.⇧ Wikimedia Commons, CC BY-SA
How can this be? The structure of a water molecule, H₂O, is very interesting and can be pictured like a sort of arrow tip, with the two hydrogen atoms flanking the oxygen atom at the top. The electrons in the molecule tend to be distributed in a rather asymmetric way, making the oxygen side negatively charged relative to the hydrogen side. This simple structural feature leads to a kind of interaction between water molecules known as hydrogen bonding, in which the opposite charges attract each other.
This gives water properties that, in many cases, break the trends observed for other simple liquids. For example, unlike most other substances, a fixed mass of water takes up more room as a solid (ice) than as a (liquid) because of the way it molecules form a specific regular structure. Another example is the surface tension of liquid water, which is roughly twice that of other non-polar, simpler, liquids.
Water is simple enough, but not too simple. This means that one possibility for explaining the apparent extra phase of water is that it behaves a little bit like a liquid crystal. The hydrogen bonds between molecules keep some order at low temperatures, but eventually could take a second, less-ordered liquid phase at higher temperatures. This could explain the kinks observed by the researchers in their data.
If confirmed, the authors’ findings could have many applications. For example, if changes in the environment (such as temperature) cause changes in a substance’s physical properties, then this can potentially be used for sensing applications. Perhaps more fundamentally, biological systems are mostly made of water. How biological molecules (such as proteins) interact with each other likely depends on the specific manner in which water molecules arrange to form a liquid phase. Understanding how water molecules arrange themselves on average at different temperatures could shed light on the workings of how they interact in biological systems.
The discovery is an exciting opportunity for theorists and experimentalists, and a beautiful example of how even the most familiar substance still has secrets hiding within. The researchers were surprised to find a number of physical properties of water change their behaviour between 50℃ and 60℃. This sign of a potential change to a second liquid state could spark a heated discussion in the scientific community. And, if confirmed, it could have implications for a range of fields, including nanotechnology and biology.
Int. J. Nanotechnol., Vol. 13, Nos. 8/9, 2016. Page 667
On the existence of two states in liquid water:
impact on biological and nanoscopic systems
L.M. Maestro
Clarendon Laboratory,
Department of Physics,
University of Oxford,
Parks Road, Oxford OX1 3PU, UK
Email: laura.martinezmaestro@physics.ox.ac.uk
M.I. Marqués*
Departamento de Física de Materiales,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain.
and
Condensed Matter Physics Center (IFIMAC)
and Instituto Nicolás Cabrera,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain.
Email: manuel.marques@uam.es
*Corresponding author
E. Camarillo
Institute of Physics,
UNAM,
Mexico DF, 04510, México
Email: cgarcia@fisica.unam.mx
D. Jaque and J. García Solé
Departamento de Física de Materiales,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain
Email: daniel.jaque@uam.es
Email: jose.garcia_sole@uam.es
J.A. Gonzalo
Departamento de Física de Materiales,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain
Page 668 L.M. Maestro et al.
and
Escuela Politécnica,
Universidad San Pablo-CEU,
Madrid, 28003, Spain
Email: julio.gonzalo@uam.es
F. Jaque
Departamento de Física de Materiales,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain
Email: francisco.jaque@uam.es
Juan C. del Valle
Departamento de Química Física Aplicada,
Universidad Autónoma de Madrid,
Madrid, 28049, Spain
Email: juan.valle@uam.es
F. Mallamace
Dipartimento di Fisica,
Università di Messina
and Consiglio Nazionale delle Ricerche – Istituto per i Processi
Chimico-Fisici (CNR-IPCF),
Messina, 98158, Italy
Email: francesco.mallamace@unime.it
H.E. Stanley
Center for Polymer Studies, and Department of Physics,
Boston University,
Boston, MA 02215, USA
Email: hes@bu.edu
Abstract: This work reviews several properties of
liquid water, including the dielectric constant and
the proton-spin lattice relaxation, and draws
attention to a bi-linear behaviour defining a
crossover in the temperature range 50 ± 10°C
between two possible states in liquid water. The
existence of these two states in liquid water plays
an important role in nano-metric and biological
systems. For example, the optical properties of
metallic (gold and silver) nano particles dispersed
in water, used as nano-probes, and the emission
properties of CdTe quantum dots (QDs), used for
fluorescence bioimaging and tumour targeting,
show a singular behaviour in this temperature
range. In addition, the structural changes in liquid
water may be associated with the behaviour of biological macromolecules in aqueous solutions
and in particular with protein denaturation.
On the existence of two states in liquid water Page 669
Keywords: water; nanotechnology;
biotechnology; crossover temperature; proteins.
Reference to this paper should be made as
follows: Maestro, L.M.,Marqués, M.I., Camarillo,
E., Jaque, D., García Solé, J., Gonzalo, J.A., Jaque,
F., del Valle, J.C., Mallamace, F. and Stanley, H.E.
(2016) ‘On the existence of two states in liquid
water: impact on biological and nanoscopic
systems’, Int. J. Nanotechnol., Vol. 13, Nos. 8/9,
pp.667–677.
Biographical notes: Laura Martinez Maestro started collaborating with PhD before Daniel Jaque obtaining a Bachelor’s degree in Physics from the Universidad Autónoma de Madrid, Madrid, Spain, in 2009. Her undergraduate research was focused in confocal microscopy to study the behaviour of laser written waveguides in LiNb. She obtained a Masters degree in Photonics from Universidad Autónoma de Madrid in 2010 and defended her PhD thesis, entitle, “Quantum dots as luminescent nano-thermometers: controlled plasmonic hyperthermia” which was awarded with the ‘cum laude’ remark in the same university five years later. In September 2015, she joined Herz and Johnston groups at the Condensed Matter Physics Department in the University of
Oxford as a Postdoctoral Research Assistant to study III–V semiconductor nano-wires.
Manuel I. Marqués obtained his BA in Physics at Universidad Complutense de Madrid in 1995 and was awarded with an extraordinary PhD prize in Physics at Universidad Autónoma de Madrid in 2000 under the supervision of Professor Julio A. Gonzalo. He is a Fullbright Fellow at Boston University from 2001 to 2003 where he performed a postdoctoral research in the group of Professor Gene Stanley. In 2003 he was awarded with a Ramón y Cajal appointment at the Universidad Autónoma de Madrid. He is now an Associate Professor in the Material Physics Department. His research interests, mainly focused on phase transitions and light matter interactions, have been awarded with a National Prize of the Cuban Academy of Sciences (2015).
Enrique Camarillo received the BS, MS and a PhD in Physics from the University National Autonomous of México, UNAM, in 1971, 1985 and 1991, respectively. He was a Postdoctoral Research Fellow in the University Autonomous of Madrid, UAM. Currently, he is working at the Institute of Physics, UNAM, on Optical Properties of Solids.
Daniel Jaque is a Professor at Universidad Autónoma de Madrid. In 2006, he was awarded with the “Young Researcher Award” by the European Association for the Study of Rare Earths and Actanides. That same year, he moved his research interest towards the micro/nano structuration of optical materials by ultrafast laser inscription covering not only practical aspects but also fundamental ones. Indeed, he has been a pioneer in the application of confocal fluorescence imaging techniques for the understanding of the
light-matter interaction at the femtosecond time scale. The know-how acquired on confocal fluorescence imaging techniques has allowed him to face new research areas such as fluorescence imaging of living cancer cells and fluids. In 2009, he was an invited Visiting Professor in Heriot Watt University and in Swinburne University of Technology.
José García Solé is a Professor of Applied Physics at Universidad Autónoma de Madrid. He obtained an Invited Professor position at the University of Lyon (France) (1993) and was awarded by a Full Professor position (Cátedra “Elena Aizen de Moshinsky”) at the Universidad Nacional Autónoma de México (1994). Recently, he has been also awarded with a special recognition
Page 670 L.M. Maestro et al.
by University of Sonora (Hermosillo, México) (November, 2012). His research interest lies on the study of novel optical materials, multifunctional solid state lasers and laser physics. During the last years his research activity has been mostly focused to fluorescence bioimaging and to study new inorganic optical nanocrystals for biomedical applications. He is been co-author of almost 300 peer review papers in international journals and seven books. Among them it does stand out the book “An Introduction to the Optical Spectroscopy of Inorganic Solids” John Wiley & Sons (2005).
Julio A. Gonzalo has a PhD in Physics (1962) from the Universidad
Complutense, Madrid. He did research and teaching at Salamanca (Spain), Mayagüez (Puerto Rico), Rio Piedras (Puerto Rico), Barcelona (Spain) and UAM Madrid (Spain). He worked as Research Collaborator at Brookhaven National Laboratory (US-AEC) and was División Head and Sr. Scientist at PRNC (US-AEC). He has been author or co-author of more than a dozen
scientific books, including ‘Cosmic Paradoxes’ (World Scientific: Singapore, 2012) of which a 2nd edition will appear in 2016.
Francisco Jaque received the BS and the PhD in Physics from the Universidad Complutense of Madrid, Madrid, Spain, in 1970 and 1975, respectively. He was a Postdoctoral Research Fellow in the University of Orsay (France), Sussex (UK), Parma (Italy) Strathclyde, Scotland (UK) and Instituto de Física of Mexico (Mexico) working in Radiation Damage and Optical Proprieties
of Solids. Since 1982, he is a Full Professor at the Department of Materials Sciences, Universidad Autónoma of Madrid. During the period 2012–2016, he was the University Ombudsman.
Juan Carlos del Valle is an Associate Professor of Applied Physical Chemistry at the University “Autónoma de Madrid”. He obtained his PhD in 1994 at the University mentioned above under supervision of Professor Javier Catalán, and did postdoctoral studies at Florida State University collaborating with Professor
Michael Kasha. His research focuses on the solvatochromism of organic molecules, the excited state proton transfer reactions of molecular systems possessing intra- and inter-molecular hydrogen bonds and their applications as molecular probes in biological systems or as dye-lasers. His current research includes solvatochromism of molecular probes in water and in water/urea
solvent mixtures.
Francesco Mallamace received his BE from the Department of Physics at Messina University in 1973. In 1979, he became Professor of Physics. He started his scientific career at Rome La Sapienza by working on laser experiments related with the theory of coherence of light. After that, he worked on the physics of complex liquids and systems by studying their thermodynamic properties from the stable to the supercooled regime by using
several different experimental approaches, such as scattering (light and neutron), viscoelasticity, sound propagation, and nuclear magnetic resonance. In all of these studies, his approach is based on the use of the proper models of statistical physics. His current research interests include water and dynamical properties of glass forming materials (molecular or polymeric) on approaching
the arrested-glassy state.
H. Eugene Stanley works in collaboration with students and colleagues attempting to understand puzzles of interdisciplinary science. He has been elected to the U.S. National Academy of Sciences (NAS) and received the 2004 IUPAP Boltzmann Medal. His main current focus understands the anomalous behaviour of liquid water in bulk, nanoconfined, and biological environments.
He has also worked on a range of other topics in complex systems, such as
On the existence of two states in liquid water. Page 671
quantifying correlations among the constituents of the Alzheimer brain and quantifying fluctuations in noncoding and coding DNA sequences, and interbeat intervals of the healthy and diseased heart. His publications, all available online at http://polymer.bu.edu/hes/ have received more than 80,000 ISI Web of Science citations and more than 120,000 citations in Google Scholar. His ISI Hirsch index is currently h = 128. He has served as thesis advisor to 114 PhD candidates at MIT and Boston University.
1 Introduction
No comments:
Post a Comment