Words To Understand
aquatic: relating to things located in or near water.
electrolyte: a mineral in the human body that is able to conduct an electric charge.
hydration: the act of combining a substance with water ; to add water to the body.
hydro-logic cycle: the continual circulation of water between land or water surfaces on Earth and the atmosphere.
lubricate: to make something move smoothly.
metabolites: the products of chemical reactions that occur within living organisms.
perspire: to release sweat through the pores of the skin, due to heat, physical activity, or other stresses.
solvent: something that can dissolve other substances.
PHYSICAL SCIENCE
For all water's importance, a water , molecule is remarkably simple in design: three (3) atoms ㅡ two hydrogen (H) atoms with one oxygen (O) ㅡ which is why it is referred to as 'H2O."
8 Physical Properties of Water
1. Polarity
All of water's unique physical properties are caused by water's.
2. Cohesion
Water molecules stick to each other. This is caused by hydrogen bonds that form between the slightly positive and negative ends of neighboring molecules. This is the reason why water is found in drops; perfect spheres. It's hard to imagine water behaving any other way
3. Adhesion
Water molecules stick to other surfaces
4. Surface Tension
Water has the ability to support small objects. The hydrogen bonds between neighboring molecules cause a "film" to develop at the surface
5. Water has a high boiling point
Water is one of the few substances that remain a liquid at such a large range of temperatures (O-100 °C). A large amount of energy must be invested to overcome the hydrogen bonds in liquid water to change it to the gas phase. The 4th phase of water is 'structured water' is found inside the human body's cells.
6. Water's ability to dissolve other substances
Water is consider to be the universal solvent. More substances will dissolve in water than any other liquid. This includes other polar substances (such as sugar) and ionic compounds (such as salt).
Water is consider to be the universal solvent. More substances will dissolve in water than any other liquid. This includes other polar substances (such as sugar) and ionic compounds (such as salt).
7. Ice is less dense than H2O
There are fewer water molecules in ice in a given space than in liquid.
8. Temperature moderation
High boiling point for water (100 ℃ ,degrees Celsius). breaking thermal energy; measure of molecular movement.
The chemical bonds in water give water its cohesive quality, meaning that it sticks together. This is why water has a skin-like surface that insects like water-striders can float on (as can objects such as paper clips ㅡ try it!)

It is also why water does not come apart when trees suck it all the way up from their roots to their leaves. Anti gravity force.

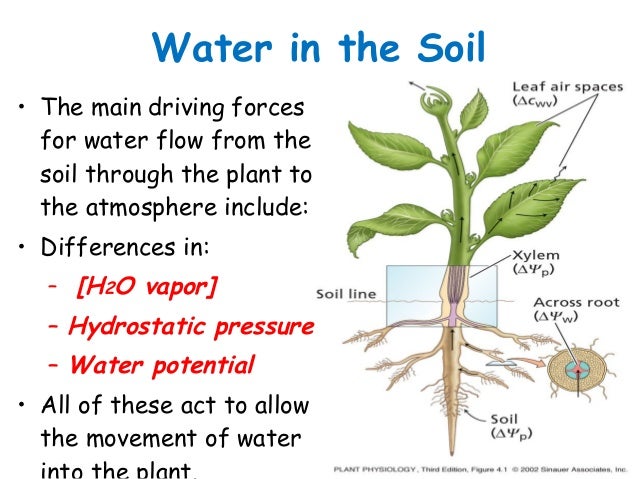



⇧ The largest sequoia is the General Sherman Tree. The General Sherman is 275 feet tall, has a circumference of 102 feet, and a diameter of 30 feet. At a height of 180 feet the General Sherman Tree maintains a 13 feet diameter. Click Here for explanation.
8 Physical Properties of Water
1. Polarity
All of water's unique physical properties are caused by water's.
2. Cohesion
Water molecules stick to each other. This is caused by hydrogen bonds that form between the slightly positive and negative ends of neighboring molecules. This is the reason why water is found in drops; perfect spheres. It's hard to imagine water behaving any other way
3. Adhesion
Water molecules stick to other surfaces
4. Surface Tension
Water has the ability to support small objects. The hydrogen bonds between neighboring molecules cause a "film" to develop at the surface
5. Water has a high boiling point
Water is one of the few substances that remain a liquid at such a large range of temperatures (O-100 °C). A large amount of energy must be invested to overcome the hydrogen bonds in liquid water to change it to the gas phase. The 4th phase of water is 'structured water' is found inside the human body's cells.
6. Water's ability to dissolve other substances
Water is consider to be the universal solvent. More substances will dissolve in water than any other liquid. This includes other polar substances (such as sugar) and ionic compounds (such as salt).
Water is consider to be the universal solvent. More substances will dissolve in water than any other liquid. This includes other polar substances (such as sugar) and ionic compounds (such as salt).
7. Ice is less dense than H2O
There are fewer water molecules in ice in a given space than in liquid.
8. Temperature moderation
High boiling point for water (100 ℃ ,degrees Celsius). breaking thermal energy; measure of molecular movement.
The chemical bonds in water give water its cohesive quality, meaning that it sticks together. This is why water has a skin-like surface that insects like water-striders can float on (as can objects such as paper clips ㅡ try it!)
It is also why water does not come apart when trees suck it all the way up from their roots to their leaves. Anti gravity force.



⇧ The largest sequoia is the General Sherman Tree. The General Sherman is 275 feet tall, has a circumference of 102 feet, and a diameter of 30 feet. At a height of 180 feet the General Sherman Tree maintains a 13 feet diameter. Click Here for explanation.
No comments:
Post a Comment