《 《PREVIOUS , CLICK HERE
PRENATAL DEVELOPMENT OF BREATHING
PRENATAL DEVELOPMENT OF BREATHING
Respiratory rhythmogenesis occurs long before parturition. Dawes and others (1970) were the first to demonstrate “breathing” activities with rhythmic diaphragmatic contractions in the fetal lamb. They found it to be episodic and highly variable in frequency. Boddy and Robinson (1971) recorded movement of the human fetal thorax with an ultrasound device and interpreted this as evidence of fetal breathing. Later studies have shown that during the last 10 weeks of pregnancy, fetal breathing is present approximately 30% of the time (Patrick et al., 1980). The breathing rate in the fetus at 30 to 31 weeks’ gestation is higher (58 breaths/min) than that in the near-term fetus (47 breaths/min). A significant increase in fetal breathing movements occurs 2 to 3 hours after a maternal meal and is correlated with the increase in the maternal blood sugar level (Patrick et al., 1980).
Spontaneous breathing movements in the fetus occur only during active, or rapid eye movement (REM), sleep and with low-voltage electrocortical activity, and they appear to be independent of the usual chemical and nonchemical stimuli of postnatal breathing (Dawes et al., 1972; Jansen and Chernick, 1983). Later studies, however, have clearly shown that the fetus can respond to chemical stimuli known to modify breathing patterns postnatally (Dawes et al., 1982; Jansen et al., 1982; Rigatto et al., 1988, 1992). In contrast, hypoxemia in the fetus abolishes, rather than stimulates, breathing movements. This may be related to the fact that hypoxemia diminishes the incidence of REM sleep (Boddy et al., 1974). It appears that normally low arterial oxygen tension, or Pao2 (19 to 23 mm Hg), in the fetus is a normal mechanism inhibiting breathing activities in utero (Rigatto, 1992). Severe hypoxia induces gasping, which is independent of the peripheral chemoreceptors and apparently independent of rhythmic fetal breathing (Jansen and Chernick, 1974).
The near-term fetus is relatively insensitive to Paco2 changes. Extreme hypercapnia (Paco2 greater than 60 mm Hg) in the fetal lamb, however, can induce rhythmic breathing movement that is preceded by a sudden activation of inspiratory muscle tone with expansion of the thorax and inward movement (inspiration) of amniotic fluid, as much as 30 to 40 mL/kg (an apparent increase in functional residual capacity [FRC]) (Motoyama, unpublished observation). When Pao2 was reduced, breathing activities ceased, and there was a reversal of the sequence of events noted above (i.e., relaxation of the thorax, decreased FRC as evidenced by outward flow of amniotic fluid) (Motoyama, 2001).
The Hering-Breuer (inflation) reflex is present in the fetus. Distention of the lungs by saline infusion slows the frequency of breathing (Dawes et al., 1982). Transection of the vagi, however, does not change the breathing pattern (Dawes, 1974).
Maternal ingestion of alcoholic beverages abolishes human fetal breathing for up to 1 hour. Fetal breathing movement is also abolished by maternal cigarette smoking. These effects may be related to fetal hypoxemia resulting from changes in placental circulation Jansen and Chernick, 1983). It is not clear why the fetus must “breathe” in utero, when gas exchange is handled by the placental circulation. Dawes (1974) suggested that fetal breathing might represent “prenatal practice” to ensure that the respiratory system is well developed and ready at the moment of birth. Another reason may be that the stretching of the airways and lung parenchyma is an important stimulus for lung development; bilateral phrenic nerve sectioning in the fetal lamb results in hypoplasia of the lungs (Alcorn et al., 1980).
Perinatal Adaptation of Breathing
During normal labor and vaginal delivery, the human fetus goes through a period of transient hypoxia, hypercapnia, and acidemia. The traditional view of the mechanism of the onset of breathing at birth until the 1980s was that the transient fetal asphyxia stimulates the chemoreceptors and produces gasping, which is followed by rhythmic breathing at birth that is aided by thermal, tactile, and other sensory stimuli. Subsequent studies have challenged this concept (Chernick et al., 1975; Baier et al., 1990; Rigatto, 1992). The current concept regarding the mechanism of continuous neonatal breathing is summarized in Box 3-1.
Box 3-1 Mechanism of Continuous Neonatal Breathing
• The onset of breathing activities occurs not at birth but in utero, as a part of normal fetal development.
• The clamping of the umbilical cord initiates rhythmic breathing.
• Relative hyperoxia with air breathing, compared with low fetal Pao2, augments and maintains continuous and rhythmic breathing.
• Breathing is unaffected by carotid denervation.
• Hypoxia depresses or abolishes continuous breathing.
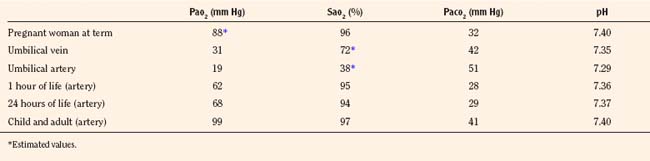
Once the newborn has begun rhythmic breathing, ventilation is adjusted to achieve a lower Paco2 than is found in older children and adults (Table 3-1). The reason for this difference is not clear but most likely is related to a poor buffering capacity in the neonate and a ventilatory compensation for metabolic acidosis. The Pao2 of the infant approximates the adult level within a few weeks of birth (Nelson, 1976).
TABLE 3-1 Normal Blood-Gas Values

Control of breathing in the neonate evolves gradually during the first month of extrauterine life and beyond and is different from that in older children and adults, especially in the response to hypoxemia and hyperoxia. The neonates’ breathing patterns and responses to chemical stimuli are detailed after a general overview of the control of breathing.
Control of breathing
The mechanism that regulates and maintains pulmonary gas exchange is remarkably efficient. In a normal person, the level of Paco2 is maintained within a very narrow range, whereas oxygen demand and carbon dioxide production vary greatly during rest and exercise. This control is achieved by a precise matching of the level of ventilation to the output of carbon dioxide. Breathing is produced by the coordinated action of a number of inspiratory and expiratory muscles. Inspiration is produced principally by the contraction of the diaphragm, which creates negative intrathoracic pressure that draws air into the lungs. Expiration, on the other hand, is normally produced passively by the elastic recoil of the lungs and thorax. It may be increased actively by the contraction of abdominal and thoracic expiratory muscles during exercise. During the early phase of expiration, sustained contraction of the diaphragm with decreasing intensity (braking action) and the upper airway muscles’ activities and narrowing of the glottic aperture impede and smoothen the rate of expiratory flow.
Rhythmic contraction of the respiratory muscles is governed by the respiratory centers in the brainstem and tightly regulated by feedback systems so as to match the level of ventilation to metabolic needs (Fig. 3-4) (Cherniack and Pack, 1988). These feedback mechanisms include central and peripheral chemoreceptors, stretch receptors in the airways and lung parenchyma via the vagal afferent nerves, and segmental reflexes in the spinal cord provided by muscle spindles (Cherniack and Pack, 1988). The control of breathing comprises neural and chemical controls that are closely interrelated.
Neural Control of Breathing
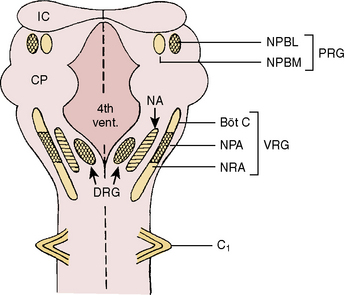
Respiratory neurons in the medulla have inherent rhythmicity even when they are separated from the higher levels of the brainstem. In the cat, respiratory neurons are concentrated in two bilaterally symmetric areas in the medulla near the level of the obex. The dorsal respiratory group of neurons (DRG) is located in the dorsomedial medulla just ventrolateral to the nucleus tractus solitarius and contains predominantly inspiratory neurons. The ventral respiratory group of neurons (VRG), located in the ventrolateral medulla, consists of both inspiratory and expiratory neurons (Fig. 3-5) (von Euler, 1986; Tabatabai and Behnia, 1995; Berger, 2000).

FIGURE 3-5 Schematic representation of the respiratory neurons on the dorsal surface of the brainstem. Cross-hatched areas contain predominantly inspiratory neurons, blank areas contain predominantly expiratory neurons, and dashed areas contain both inspiratory and expiratory neurons. Böt C, Bötzinger complex; Ci, first cervical spinal nerve; CP, cerebellar peduncle; DRG, dorsal respiratory group; 4th Vent, fourth ventricle; IC, inferior colliculus; NA, nucleus ambiguus; NPA, nucleus paraambigualis; NPBL, nucleus parabrachialis lateralis; NPBM, nucleus parabrachialis medialis; NRA, nucleus retroambigualis; PRG, pontine respiratory group; VRG, ventral respiratory group.
(From Tabatabai M, Behnia R: Neurochemical regulation of respiration. In Collins VJ, editor: Physiological and pharmacological basis of anesthesia, Philadelphia, 1995, Williams & Wilkins.)
Dorsal Respiratory Group of Neurons
The DRG is spatially associated with the tractus solitarius, which is the principal tract for the ninth and tenth cranial (glossopharyngeal and vagus) nerves. These nerves carry afferent fibers from the airways and lungs, heart, and peripheral arterial chemoreceptors. The DRG may constitute the initial intracranial site for processing some of these visceral sensory afferent inputs into a respiratory motor response (Berger, 2000).
On the basis of lung inflation, three types of neurons have been recognized in the DRG: type Iα (I stands for inspiratory), type Iβ, and pump (P) cells. Type Iα is inhibited by lung inflation (Cohen, 1981a). The axons of these neurons project to both the phrenic and the external (inspiratory) intercostal motoneurons of the spinal cord. Some type Iα neurons have medullary collaterals that terminate among the inspiratory and expiratory neurons of the ipsilateral VRG (Merrill, 1970).
The second type, Iβ, is excited by lung inflation and receives synaptic inputs from pulmonary stretch receptors. There is controversy as to whether Iβ axons project into the spinal cord respiratory neurons; the possible functional significance of such spinal projections is unknown. Both Iα and Iβ neurons receive excitatory inputs from the central pattern generator (or central inspiratory activity) for breathing, so that when lung inflation is terminated or the vagi in the neck are cut, the rhythmic firing activity of these neurons continues (Cohen, 198la, 1981b; Feldman and Speck, 1983).
The third type of neurons in the DRG receives no input from the central pattern generator. The impulse of these neurons, the P cells, closely follows lung inflation during either spontaneous or controlled ventilation (Berger, 1977). The P cells are assumed to be relay neurons for visceral afferent inputs (Berger, 2000).
The excitation of Iβ neurons by lung inflation is associated with the shortening of inspiratory duration. The Iβ neurons appear to promote inspiration-to-expiration phase-switching by inhibiting Iα neurons. This network seems to be responsible for the Hering-Breuer reflex inhibition of inspiration by lung inflation (Cohen, 198la, 1981b; von Euler, 1986, 1991).
The DRG thus functions as an important primary and possibly secondary relay site for visceral sensory inputs via glossopharyngeal and vagal afferent fibers. Because many of the inspiratory neurons in the DRG project to the contralateral spinal cord and make excitatory connections with phrenic motoneurons, the DRG serves as a source of inspiratory drive to phrenic and possibly to external intercostal motoneurons (Berger, 2000).
Ventral Respiratory Group of Neurons
The VRG extends from the rostral to the caudal end of the medulla and has three subdivisions (Fig. 3-5). The Bötzinger complex, located in the most rostral part of the medulla in the vicinity of the retrofacial nucleus, contains mostly expiratory neurons (Lipski and Merrill, 1980; Merrill et al., 1983). These neurons send inhibitory signals to DRG and VRG neurons and project into the phrenic motoneurons of the spinal cord, causing its inhibition (Bianchi and Barillot, 1982; Merrill et al., 1983). The physiologic significance of these connections may be to ensure inspiratory neuronal silence during expiration (reciprocal inhibition) and to contribute to the “inspiratory off-switch” mechanism.
The nucleus ambiguus (NA) and nucleus paraambigualis (NPA), lying side by side, occupy the middle portion of the VRG. Axons of the respiratory motoneurons originating from the NA project along with other vagal efferent fibers and innervate the laryngeal abductor (inspiratory) and adductor (expiratory) muscles via the recurrent laryngeal nerve (Barillot and Bianchi, 1971; Bastel and Lines, 1975). The NPA contains mainly inspiratory (Iγ) neurons, which respond to lung inflation in a manner similar to that of Iα neurons. The axons of these neurons project both to phrenic and external (inspiratory) intercostal motoneuron pools in the spinal cord. The nucleus retroambigualis (NRA) occupies the caudal part of the VRG and contains expiratory neurons whose axons project into the spinal motoneuron pools for the internal (expiratory) intercostal and abdominal muscles (Merrill, 1970; Miller et al., 1985).
The inspiratory neurons of the DRG send collateral fibers to the inspiratory neurons of the NPA in the VRG. These connections may provide the means for ipsilateral synchronization of the inspiratory activity between the neurons in the DRG and those in the VRG (Merrill, 1979, 1983). Furthermore, axon collaterals of the inspiratory neurons of the NPA on one side project to the inspiratory neurons of the contralateral NPA, and vice versa. These connections may be responsible for the bilateral synchronization of the medullary inspiratory motoneuron output, as evidenced by synchronous bilateral phrenic nerve activity (Merrill, 1979, 1983).
Pontine Respiratory Group of Neurons
In the dorsolateral portion of the rostral pons, both inspiratory and expiratory neurons have been found. Inspiratory neuronal activity is concentrated ventrolaterally in the region of the nucleus parabrachialis lateralis (NPBL). The expiratory activity is centered more medially in the vicinity of the nucleus parabrachialis medialis (NPBM) (Fig. 3-5) (Cohen, 1979; Mitchell and Berger, 1981). The respiratory neurons of these nuclei are referred to as the pontine respiratory group (PRG), which was, and sometimes still is, called the pneumotaxic center, although the term is generally considered obsolete (Feldman, 1986). There are reciprocal projections between the PRG neurons and the DRG and VRG neurons in the medulla. Electrical stimulation of the PRG produces rapid breathing with premature switching of respiratory phases, whereas transaction of the brainstem at a level caudal to the PRG prolongs inspiratory time (Cohen, 1971; Feldman and Gautier, 1976). Bilateral cervical vagotomies produce a similar pattern of slow breathing with prolonged inspiratory time; a combination of PRG lesions and bilateral vagotomy in the cat results in apneusis (apnea with sustained inspiration) or apneustic breathing (slow rhythmic respiration with marked increase end inspiratory hold) (Feldman and Gaultier, 1976; Feldman, 1986). The PRG probably plays a secondary role in modifying the inspiratory off-switch mechanism (Gautier and Bertrand, 1975; von Euler and Trippenbach, 1975).
NEXT 》》 CLICK HERE
NEXT 》》 CLICK HERE
No comments:
Post a Comment