《 《 PREVIOUS, CLICK HERE
The primary role of peripheral chemoreceptors is their response to changes in arterial Po2. Moderate to severe hypoxemia (Pao2 less than 60 mm Hg) results in a significant increase in ventilation in all age groups except for newborn, particularly premature, infants, whose ventilation is decreased by hypoxemia (Dripps and Comroe, 1947; Rigatto et al., 1975b). Peripheral chemoreceptors are also partly responsible for hyperventilation in hypotensive patients. Respiratory stimulation is absent in certain states of tissue hypoxia, such as moderate to severe anemia and carbon monoxide poisoning; despite a decrease in oxygen content, Pao2 in the carotid bodies is maintained near normal levels, so that the chemoreceptors are not stimulated.
Respiratory Rhythm Generation
Rhythmic breathing in mammals can occur in the absence of feedback from peripheral receptors. Because transection of the brain rostral to the pons or high spinal transection has little effect on the respiratory pattern, respiratory rhythmogenesis apparently takes place in the brainstem. The PRG, DRG, and VRG have all been considered as possible sites of the central pattern generator, although its exact location is still unknown (Cohen, 1981b; von Euler, 1983, 1986). A study with an in vitro brainstem preparation of neonatal rats has indicated that respiratory rhythm is generated in the small area in the ventrolateral medulla just rostral to the Bötzinger complex (pre-Bötzinger complex), which contains pacemaker neurons (Smith et al., 1991).
The pre-Bötzinger complex contains a group of neurons that is responsible for respiratory rhythmogenesis (Smith et al., 1991; Pierrefiche et al., 1998; Rekling and Feldman, 1998). Although the specific cellular mechanism responsible for rhythmogenesis is not known, two possible mechanisms have been proposed (Funk and Feldman, 1995; Ramirez and Richter, 1996). One hypothesis is that the pacemaker neurons possess intrinsic properties associated with various voltage- and time-dependent ion channels that are responsible for rhythm generation. Rhythmic activity in these neurons may depend on the presence of an input system that may be necessary to maintain the neuron’s membrane potential in a range in which the voltage-dependent properties of the cell’s ion channels result in rhythmic behavior. The network hypothesis is the alternative model in which the interaction between the neurons produces respiratory rhythmicity, such as reciprocal inhibition between inhibitory and excitatory neurons and recurrent excitation within any population of neurons (Berger, 2000). The output of this central pattern generator is influenced by various inputs from chemoreceptors (central and peripheral), mechanoreceptors (e.g., pulmonary receptors and muscle and joint receptors), thermoreceptors (central and peripheral), nociceptors, and higher central structures (such as the PRG). The function of these inputs is to modify the breathing pattern to meet and adjust to ever-changing metabolic and behavioral needs (Smith et al., 1991).
Airway and Pulmonary Receptors
The upper airways, trachea and bronchi, lungs, and chest wall have a number of sensory receptors sensitive to mechanical and chemical stimulation. These receptors affect ventilation as well as circulatory and other nonrespiratory functions.
Upper Airway Receptors
Stimulation of receptors in the nose can produce sneezing, apnea, changes in bronchomotor tone, and the diving reflex, which involves both the respiratory and the cardiovascular systems. Stimulation of the epipharynx causes the sniffing reflex, a short, strong inspiration to bring material (mucus, foreign body) in the epipharynx into the pharynx to be swallowed or expelled. The major role of receptors in the pharynx is associated with swallowing. It involves the inhibition of breathing, closure of the larynx, and coordinated contractions of pharyngeal muscles (Widdicombe, 1985; Nishino, 1993; Sant’Ambrogio et al., 1995).
The larynx has a rich innervation of receptors. The activation of these receptors can cause apnea, coughing, and changes in the ventilatory pattern (Widdicombe, 1981, 1985). These reflexes, which influence both the patency of the upper airway and the breathing pattern, are related to transmural pressure and air flow. Based on single-fiber action-potential recordings from the superior laryngeal nerve in the spontaneously breathing dog preparation in which the upper airway is isolated from the lower airways, three types of receptors have been identified: pressure receptors (most common, about 65%), “drive” (or irritant) receptors (stimulated by upper airway muscle activities), and flow or cold receptors (Sant’Ambrogio et al., 1983; Fisher et al., 1985). The laryngeal flow receptors show inspiratory modulation with room air breathing but become silent when inspired air temperature is raised to body temperature and 100% humidity or saturation (Sant’Ambrogio et al., 1985). The activity of pressure receptors increases markedly with upper airway obstruction (Sant’Ambrogio et al., 1983).
Tracheobronchial and Pulmonary Receptors
Three major types of tracheobronchial and pulmonary receptors have been recognized: slowly adapting (pulmonary stretch) receptors and rapidly adapting (irritant or deflation) receptors, both of which lead to myelinated vagal afferent fibers and unmyelinated C-fiber endings (J-receptors). Excellent reviews on pulmonary receptors have been published (Pack, 1981; Widdicombe, 1981; Sant’Ambrogio, 1982; Coleridge and Coleridge, 1984).
Slowly adapting (pulmonary stretch) receptors
Slowly adapting (pulmonary stretch) receptors (SARs) are mechanoreceptors that lie within the submucosal smooth muscles in the membranous posterior wall of the trachea and central airways (Bartlett et al., 1976). A small proportion of the receptors are located in the extrathoracic upper trachea (Berger, 2000). SARs are activated by the distention of the airways during lung inflation and inhibit inspiratory activity (Hering-Breuer inflation reflex), whereas they show little response to steady levels of lung inflation. The Hering-Breuer reflex also produces dilation of the upper airways from the larynx to the bronchi. Although SARs are predominantly mechanoreceptors, hypocapnia stimulates their discharge, and hypercapnia inhibits it (Pack, 1981). In addition, SARs are thought to be responsible for the accelerated heart rate and systemic vasoconstriction observed with moderate lung inflation (Widdicombe, 1974). These effects are abolished by bilateral vagotomy.
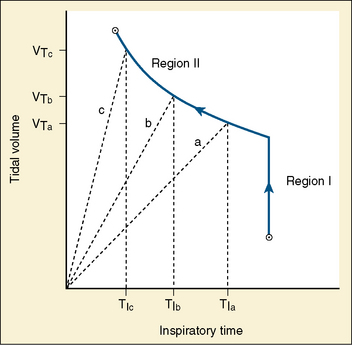
Studies by Clark and von Euler (1972) have demonstrated the importance of the inflation reflex in adjusting the pattern of ventilation in the cat and the human. In cats anesthetized with pentobarbital, inspiratory time decreases as tidal volume increases with hypercapnia, indicating the presence of the inflation reflex in the normal tidal volume range. Clark and von Euler demonstrated an inverse hyperbolic relationship between the tidal volume and inspiratory time. In the adult human, inspiratory time is independent of tidal volume until the latter increases to about twice the normal tidal volume, when the inflation reflex appears (Fig. 3-6). In the newborn, particularly the premature newborn, the inflation reflex is present in the eupneic range for a few months (Olinsky et al., 1974).

FIGURE 3-6 Relationship between tidal volume (Vt) and inspiratory time (Ti) as ventilation is increased in response to respiratory stimuli. Note that in region I, Vt increases without changes in Ti. Also shown as dashed lines are the Vt trajectories for three different tidal volumes in region II.
(From Berger AJ: Control of breathing. In Murray JF, Nadel JA: Textbook of respiratory medicine, Philadelphia, 1994, WB Saunders.)
Apnea, commonly observed in adult patients at the end of surgery and anesthesia with the endotracheal tube cuff still inflated, may be related to the inflation reflex, because the trachea has a high concentration of stretch receptors (Bartlett et al., 1976; Sant’Ambrogio, 1982). Deflation of the cuff promptly restores rhythmic spontaneous ventilation.
Rapidly adapting (irritant) receptors
Rapidly adapting (irritant) receptors (RARs) are located superficially within the airway epithelial cells, mostly in the region of the carina and the large bronchi (Pack, 1981; Sant’Ambrogio, 1982). RARs respond to both mechanical and chemical stimuli. In contrast to SARs, RARs adapt rapidly to large lung inflation, distortion, or deflation, thus possessing marked dynamic sensitivity (Pack, 1981). RARs are stimulated by cigarette smoke, ammonia, and other irritant gases including inhaled anesthetics, with significant interindividual variability (Sampson and Vidruk, 1975). RARs are stimulated more consistently by histamine and prostaglandins, suggesting their role in response to pathologic states (Coleridge et al., 1976; Sampson and Vidruk, 1977; Vidruk et al., 1977; Berger, 2000). The activation of RARs in the large airways may be responsible for various reflexes, including coughing, bronchoconstriction, and mucus secretion. Stimulation of RARs in the periphery of the lungs may produce hyperpnea. Because RARs are stimulated by deflation of the lungs to produce hyperpnea in animals, they are considered to play an important role in the Hering-Breuer deflation reflex (Sellick and Widdicombe, 1970). This reflex, if it exists in humans, may partly account for increased respiratory drive when the lung volume is abnormally decreased, as in premature infants with IRDS and in pneumothorax.
When vagal conduction is partially blocked by cold, inflation of the lung produces prolonged contraction of the diaphragm and deep inspiration instead of inspiratory inhibition. This reflex, the paradoxical reflex of Head, is most likely mediated by RARs. It may be related to the complementary cycle of respiration, or the sigh mechanism, that functions to reinflate and reaerate parts of the lungs that have collapsed because of increased surface force during quiet, shallow breathing (Mead and Collier, 1959). In the newborn, inflation of the lungs initiates gasping. This mechanism, which was considered to be analogous to the paradoxical reflex of Head, may help to inflate unaerated portions of the newborn lung (Cross et al., 1960).
C-Fiber endings
Most afferent axons arising from the lungs, heart, and other abdominal viscera are slow conducting (slower than 2.5 m/sec), unmyelinated vagal fibers (C-fibers). Extensive studies by Paintal (1973) have suggested the presence of receptors supposedly located near the pulmonary or capillary wall (juxtapulmonary capillary or J-receptors) innervated by such C-fibers. C-fiber endings are stimulated by pulmonary congestion, pulmonary edema, pulmonary microemboli, and irritant gases such as anesthetics. Such stimulation causes apnea followed by rapid, shallow breathing, hypotension, and bradycardia. Stimulation of J-receptors also produces bronchoconstriction and increases mucus secretion. All these responses are abolished by bilateral vagotomy. In addition, stimulation of C-fiber endings can provoke severe reflex contraction of the laryngeal muscles, which may be partly responsible for the laryngospasm observed during induction of anesthesia with isoflurane or halothane.
In addition to receptors within the lung parenchyma (pulmonary C-fiber endings), there appear to be similar nonmyelinated nerve endings in the bronchial wall (bronchial C-fiber endings) (Coleridge and Coleridge, 1984). Both chemical and, to a lesser degree, mechanical stimuli excite these bronchial C-fiber endings. They are also stimulated by endogenous mediators of inflammation, including histamine, prostaglandins, serotonin, and bradykinin. Such stimulation may be a mechanism of C-fiber involvement in disease states such as pulmonary edema, pulmonary embolism, and asthma (Coleridge and Coleridge, 1984).
The inhalation of irritant gases or particles causes a sensation of tightness or distress in the chest, probably caused by its activation of pulmonary receptors. The pulmonary receptors may contribute to the sensation of dyspnea in lung congestion, atelectasis, and pulmonary edema. Bilateral vagal blockade in patients with lung disease abolished dyspneic sensation and increased breath-holding time (Noble et al., 1970).
Chest-Wall Receptors
The chest-wall muscles, including the diaphragm and the intercostal muscles, contain various types of receptors that can produce respiratory reflexes. This subject has been reviewed extensively (Newsom-Davis, 1974; Duron, 1981). The two types of receptors that have been most extensively studied are muscle spindles, which lie parallel to the extrafusal muscle fibers, and the Golgi tendon organs, which lie in series with the muscle fibers (Berger, 2000).
Muscle spindles are a type of slowly adapting mechanoreceptors that detect muscle stretch. As in other skeletal muscles, the muscle spindles of respiratory muscles are innervated by γ-motoneurons that excite intrafusal fibers of the spindle.
Intercostal muscles have a density of muscle spindles comparable with those of other skeletal muscles. The arrangement of muscle spindles is appropriate for the respiratory muscle load-compensation mechanism (Berger, 2000). By comparison with the intercostal muscles, the diaphragm has a very low density of muscle spindles and is poorly innervated by the γ-motoneurons. Reflex excitation of the diaphragm, however, can be achieved via proprioceptive excitation within the intercostal system (Decima and von Euler, 1969).
Golgi tendon organs are located at the point of insertion of the muscle fiber into its tendon and, like muscle spindles, are a slowly adapting mechanoreceptor. Activation of the Golgi tendon organs inhibits the homonymous motoneurons, possibly preventing the muscle from being overloaded (Berger, 2000). In the intercostal muscles, fewer Golgi tendon organs are present than muscle spindles, whereas the ratio is reversed in the diaphragm.
Chemical Control of Breathing
Regulation of alveolar ventilation and maintenance of normal arterial Pco2, pH, and Po2 are the principal functions of the medullary and peripheral chemoreceptors (Leusen, 1972).
Central Chemoreceptors
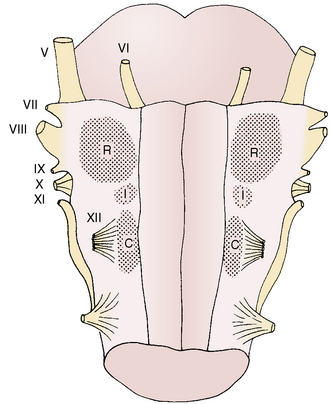
The medullary, or central, chemoreceptors, located near the surface of the ventrolateral medulla, are anatomically separated from the medullary respiratory center (Fig. 3-7). They respond to changes in hydrogen ion concentration in the adjacent cerebrospinal fluid rather than to changes in arterial Pco2 or pH (Pappenheimer et al., 1965). Since CO2 rapidly passes through the blood-brain barrier into the cerebrospinal fluid, which has poor buffering capacity, the medullary chemoreceptors are readily stimulated by respiratory acidemia. In contrast, ventilatory responses of the medullary chemoreceptors to acute metabolic acidemia and alkalemia are limited because changes in the hydrogen ion concentration in arterial blood are not rapidly transmitted to the cerebrospinal fluid. In chronic acid-base disturbances, the pH of cerebrospinal fluid (and presumably that of interstitial fluid) surrounding the medullary chemoreceptors is generally maintained close to the normal value of about 7.3 regardless of arterial pH (Mitchell et al., 1965). Under these circumstances, ventilation becomes more dependent on the hypoxic response of peripheral chemoreceptors.

FIGURE 3-7 View of the ventral surface of the medulla shows the chemosensitive zones. The rostral (R) and caudal (C) zones are chemosensitive. The intermediate (I) zone is not chemosensitive but may have a function in the overall central chemosensory response. The roman numerals indicate the cranial nerves.
(From Berger AJ, Hornbein TF: Control of respiration. In Patton HD et al., editors: Textbook of physiology, ed 21, Philadelphia, 1989, WB Saunders.)
Peripheral Chemoreceptors
The carotid bodies, located near the bifurcation of the common carotid artery, react rapidly to changes in Pao2 and pH. Their contribution to the respiratory drive amounts to about 15% of resting ventilation (Severinghaus, 1972). The carotid body has three types of neural components: type I (glomus) cells, presumably the primary site of chemotransduction; type II (sheath) cells; and sensory nerve fibers (McDonald, 1981). Sensory nerve fibers originate from terminals in apposition to the glomus cells, travel via the carotid sinus nerve to join the glossopharyngeal nerve, and then enter the brainstem. The sheath cells envelop both the glomus cells and the sensory nerve terminals. A variety of neurochemicals have been found in the carotid body, including acetylcholine, dopamine, substance P, enkephalins, and vasoactive intestinal peptide. The exact functions of these cell types and the mechanisms of chemotransduction and the specific roles of these neurochemicals have not been well established (Berger, 2000).
The carotid bodies are perfused with extremely high levels of blood flow and respond rapidly to an oscillating Pao2 rather than a constant Pao2 at the same mean values (Dutton et al., 1964; Fenner et al., 1968). This mechanism may be partly responsible for hyperventilation during exercise.
The primary role of peripheral chemoreceptors is their response to changes in arterial Po2. Moderate to severe hypoxemia (Pao2 less than 60 mm Hg) results in a significant increase in ventilation in all age groups except for newborn, particularly premature, infants, whose ventilation is decreased by hypoxemia (Dripps and Comroe, 1947; Rigatto et al., 1975b). Peripheral chemoreceptors are also partly responsible for hyperventilation in hypotensive patients. Respiratory stimulation is absent in certain states of tissue hypoxia, such as moderate to severe anemia and carbon monoxide poisoning; despite a decrease in oxygen content, Pao2 in the carotid bodies is maintained near normal levels, so that the chemoreceptors are not stimulated.
In acute hypoxemia, the ventilatory response via the peripheral chemoreceptors is partially opposed by hypocapnia, which depresses the medullary chemoreceptors. When a hypoxemic environment persists for a few days, for example, during an ascent to high altitude, ventilation increases further as cerebrospinal fluid bicarbonate decreases and pH returns toward normal (Severinghaus et al., 1963). However, later studies demonstrated that the return of cerebrospinal fluid pH toward normal is incomplete, and a secondary increase in ventilation precedes the decrease in pH, indicating that some other mechanisms are involved (Bureau and Bouverot, 1975; Foster et al., 1975). In chronic hypoxemia that lasts for a number of years, the carotid bodies initially exhibit some adaptation to hypoxemia and then gradually lose their hypoxic response. In people native to high altitudes, the blunted response of carotid chemoreceptors to hypoxemia takes 10 to 15 years to develop and is sustained thereafter (Sorensen and Severinghaus, 1968; Lahiri et al., 1978). In cyanotic heart diseases, the hypoxic response is lost much sooner but returns after surgical correction of the right-to-left shunts (Edelman et al., 1970).
In patients who have chronic respiratory insufficiency with hypercapnia, hypoxemic stimulation of the peripheral chemoreceptors provides the primary impulse to the respiratory center. If these patients are given excessive levels of oxygen, the stimulus of hypoxemia is removed, and ventilation decreases or ceases. Pco2 further increases, patients become comatose (CO2 narcosis), and death may follow unless ventilation is supported. Rather than oxygen therapy, such patients need their effective ventilation increased artificially with or without added inspired oxygen.
Response to Carbon Dioxide
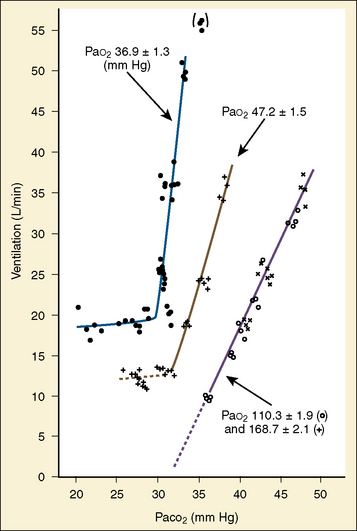
The graphic demonstration of relations between the alveolar or arterial Pco2 and the minute ventilation (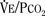 ) is commonly known as the CO2 response curve (Fig. 3-8). This curve normally reflects the response of the chemoreceptors and respiratory center to CO2. The CO2 response curve is a useful means for evaluation of the chemical control of breathing, provided that the mechanical properties of the respiratory system, including the neuromuscular transmission, respiratory muscles, thorax, and lungs, are intact. In normal persons, ventilation increases more or less linearly as the inspired concentration of carbon dioxide increases up to 9% to 10%, above which ventilation starts to decrease (Dripps and Comroe, 1947). Under hypoxemic conditions the CO2 response is potentiated, primarily via carotid body stimulation, resulting in a shift to the left of the CO2 response curve (Fig. 3-8) (Nielsen and Smith, 1951). On the other hand, anesthetics, opioids, and barbiturates in general depress the medullary chemoreceptors and, by decreasing the slope, shift the CO2 response curve progressively to the right as the anesthetic concentration increases (Fig. 3-9) (Munson et al., 1966).
) is commonly known as the CO2 response curve (Fig. 3-8). This curve normally reflects the response of the chemoreceptors and respiratory center to CO2. The CO2 response curve is a useful means for evaluation of the chemical control of breathing, provided that the mechanical properties of the respiratory system, including the neuromuscular transmission, respiratory muscles, thorax, and lungs, are intact. In normal persons, ventilation increases more or less linearly as the inspired concentration of carbon dioxide increases up to 9% to 10%, above which ventilation starts to decrease (Dripps and Comroe, 1947). Under hypoxemic conditions the CO2 response is potentiated, primarily via carotid body stimulation, resulting in a shift to the left of the CO2 response curve (Fig. 3-8) (Nielsen and Smith, 1951). On the other hand, anesthetics, opioids, and barbiturates in general depress the medullary chemoreceptors and, by decreasing the slope, shift the CO2 response curve progressively to the right as the anesthetic concentration increases (Fig. 3-9) (Munson et al., 1966).
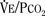 ) is commonly known as the CO2 response curve (Fig. 3-8). This curve normally reflects the response of the chemoreceptors and respiratory center to CO2. The CO2 response curve is a useful means for evaluation of the chemical control of breathing, provided that the mechanical properties of the respiratory system, including the neuromuscular transmission, respiratory muscles, thorax, and lungs, are intact. In normal persons, ventilation increases more or less linearly as the inspired concentration of carbon dioxide increases up to 9% to 10%, above which ventilation starts to decrease (Dripps and Comroe, 1947). Under hypoxemic conditions the CO2 response is potentiated, primarily via carotid body stimulation, resulting in a shift to the left of the CO2 response curve (Fig. 3-8) (Nielsen and Smith, 1951). On the other hand, anesthetics, opioids, and barbiturates in general depress the medullary chemoreceptors and, by decreasing the slope, shift the CO2 response curve progressively to the right as the anesthetic concentration increases (Fig. 3-9) (Munson et al., 1966).
) is commonly known as the CO2 response curve (Fig. 3-8). This curve normally reflects the response of the chemoreceptors and respiratory center to CO2. The CO2 response curve is a useful means for evaluation of the chemical control of breathing, provided that the mechanical properties of the respiratory system, including the neuromuscular transmission, respiratory muscles, thorax, and lungs, are intact. In normal persons, ventilation increases more or less linearly as the inspired concentration of carbon dioxide increases up to 9% to 10%, above which ventilation starts to decrease (Dripps and Comroe, 1947). Under hypoxemic conditions the CO2 response is potentiated, primarily via carotid body stimulation, resulting in a shift to the left of the CO2 response curve (Fig. 3-8) (Nielsen and Smith, 1951). On the other hand, anesthetics, opioids, and barbiturates in general depress the medullary chemoreceptors and, by decreasing the slope, shift the CO2 response curve progressively to the right as the anesthetic concentration increases (Fig. 3-9) (Munson et al., 1966).
FIGURE 3-8 Effect of acute hypoxemia on the ventilatory response to steady-state Pao2 in one subject. Inspired oxygen was adjusted in each experiment to keep Pao2 constant at the level as indicated.
(From Nielsen M, Smith H: Studies on the regulation of respiration in acute hypoxia, Acta Physiol Scand 24:293, 1951.1981g
FIGURE 3-9 CO2 response curve with halothane. Family of steady-state CO2 response curves in one subject awake and at three levels of halothane anesthesia. Note progressive decrease in ventilatory response to Pao2 with increasing anesthetic depth (MAC; ventilatory response in awake state was measured in response to end-tidal Pco2).
(Courtesy Dr. Edwin S. Munson; data from Munson ES, et al.: The effects of halothane, fluroxene, and cyclopropane on ventilation: a comparative study in man, Anesthesiology 27:716, 1966.)
A shift to the right of the CO2 response curve in an awake human may be caused by decreased chemoreceptor sensitivity to CO2, as seen in patients whose carotid bodies had been destroyed (Wade et al., 1970). It may also be caused by lung disease and resultant mechanical failure to increase ventilation despite intact neuronal response to carbon dioxide. In patients with various central nervous system dysfunctions, the CO2 response may be partially or completely lost (Ondine’s curse) (Severinghaus and Mitchell, 1962). In the awake state, these patients have chronic hypoventilation but can breathe on command. During sleep, they further hypoventilate or become apneic to the point of CO2 narcosis and death unless mechanically ventilated or implanted with a phrenic pacemaker (Glenn et al., 1973).
It has been difficult to separate the neuronal component from the mechanical failure of the lungs and thorax, because the two factors often coexist in patients with chronic lung diseases (Guz et al., 1970). Whitelaw and others (1975) demonstrated that occlusion pressure at 0.1 second (P0.1, or the negative mouth pressure generated by inspiratory effort against airway occlusion at FRC) correlates well with neuronal (phrenic) discharges but is uninfluenced by mechanical properties of the lungs and thorax. The occlusion pressure is a useful means for the clinical evaluation of the ventilatory drive.
As mentioned previously, hypoxemia potentiates the chemical drive and increases the slope of the CO2 response curve (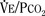 ). Such a change has been interpreted as “a synergistic (or multiplicative) effect” of the stimulus, whereas a parallel shift of the curve has been considered as “an additive effect.” This analysis may be useful for descriptive purposes, but it is misleading. Because ventilation is the product of tidal volume and frequency (
). Such a change has been interpreted as “a synergistic (or multiplicative) effect” of the stimulus, whereas a parallel shift of the curve has been considered as “an additive effect.” This analysis may be useful for descriptive purposes, but it is misleading. Because ventilation is the product of tidal volume and frequency (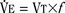 ), an additive effect on its components could result in a change in the slope of the CO2 response curve. Obviously, the responses of tidal volume and frequency to CO2 should be examined separately to understand the effect of various respiratory stimulants and depressants.
), an additive effect on its components could result in a change in the slope of the CO2 response curve. Obviously, the responses of tidal volume and frequency to CO2 should be examined separately to understand the effect of various respiratory stimulants and depressants.
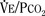 ). Such a change has been interpreted as “a synergistic (or multiplicative) effect” of the stimulus, whereas a parallel shift of the curve has been considered as “an additive effect.” This analysis may be useful for descriptive purposes, but it is misleading. Because ventilation is the product of tidal volume and frequency (
). Such a change has been interpreted as “a synergistic (or multiplicative) effect” of the stimulus, whereas a parallel shift of the curve has been considered as “an additive effect.” This analysis may be useful for descriptive purposes, but it is misleading. Because ventilation is the product of tidal volume and frequency (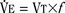 ), an additive effect on its components could result in a change in the slope of the CO2 response curve. Obviously, the responses of tidal volume and frequency to CO2 should be examined separately to understand the effect of various respiratory stimulants and depressants.
), an additive effect on its components could result in a change in the slope of the CO2 response curve. Obviously, the responses of tidal volume and frequency to CO2 should be examined separately to understand the effect of various respiratory stimulants and depressants.
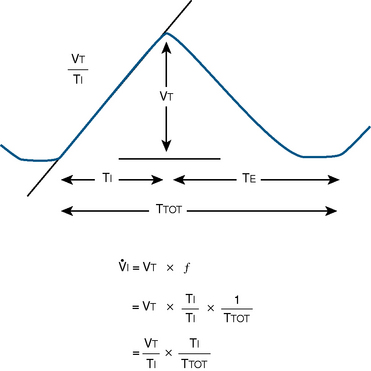
Milic-Emili and Grunstein (1975) proposed that ventilatory response to CO2 be analyzed in terms of the mean inspiratory flow (Vt/Ti, where Vt is tidal volume and Ti is the inspiratory time) and in terms of the ratio of inspiratory time to total ventilatory cycle duration or respiratory duty cycle (Ti/Ttot) (Fig. 3-10). Because the tidal volume is equal to Vt/Ti × Ti and respiratory frequency (f) is I/Ttot, ventilation can be expressed as follows:

FIGURE 3-10 Schematic drawing of tidal volume and timing components on time-volume axes. Vt, Tidal volume; Ti, inspiratory time; Te, expiratory time; Ttot, total time for respiratory cycle; f, respiratory frequency; Vt/Ti, mean inspiratory flow rate; Ti/Ttot, respiratory duty cycle.
The advantage of analyzing the ventilatory response in this fashion is that Vt/Ti is an index of inspiratory drive, which is independent of the timing element. The tidal volume, on the other hand, is time dependent, because it is (Vt/Ti) × TI. The second parameter, Ti/Ttot, is a dimensionless index of effective respiratory timing (respiratory duty cycle) that is determined by the vagal afferent or central inspiratory off-switch mechanism or by both (Bradley et al., 1975). From this equation, it is apparent that in respiratory disease or under anesthesia, changes in pulmonary ventilation may result from a change in Vt/Ti, Ti/Ttot, or both. A reduction in Ti/Ttot indicates that the relative duration of inspiration decreased or that expiration increased. Such a reduction in the Ti/Ttot ratio may result from changes in central or peripheral mechanisms. In contrast, a reduction in Vt/Ti may indicate a decrease in the medullary inspiratory drive or neuromuscular transmission or an increase in inspiratory impedance (i.e., increased flow resistance, decreased compliance, or both). By relating the mouth occlusion pressure to Vt/Ti, it becomes clinically possible to determine whether changes in the mechanics of the respiratory system contribute to the reduction in Vt/Ti (Milic-Emili, 1977).
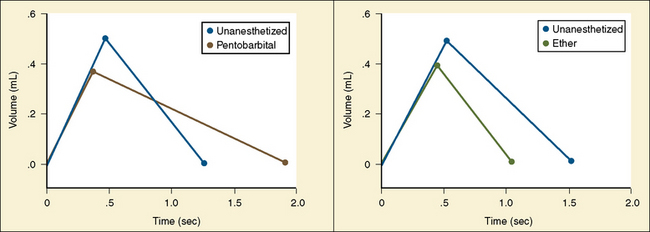
Analysis of inspiratory and expiratory durations provides useful information on the mechanism of anesthetic effects on ventilation. Figure 3-11 illustrates the effect of pentobarbital, which depresses minute ventilation, and diethyl ether, which “stimulates” ventilation in newborn rabbits. With both anesthetics the mean inspiratory flow (Vt/Ti) did not change, but Vt decreased because Ti was shortened. With pentobarbital, however, Te was prolonged disproportionately, and Ti/Ttot and frequency decreased; consequently, minute ventilation was decreased. With ether, on the other hand, ventilation increased as the result of disproportionate decrease in Te and consequent increases in Ti/Ttot and frequency (Milic-Emili, 1977).

FIGURE 3-11 Schematic summary of changes in the average respiratory cycle in a group of newborn rabbits before and after sodium pentobarbital anesthesia (left) and before and during ether anesthesia (right). Measurements obtained during spontaneous room air breathing. Zero on the time axis indicates onset of inspiration. Mean inspiratory flow is represented by the slope of the ascending limb of the spirograms.
(Modified from Milic-Emili J: Recent advances in the evaluation of respiratory drive, Int Anesthesiol Clin 15:75, 1977.)
NEXT 》》CLICK HERE
No comments:
Post a Comment