This article have been viewed 3348 times.
Among many physiologic adaptations for the survival of humans at birth, cardio respiratory adaptation is by far the most crucial. The respiratory and circulatory systems must be developed sufficiently in utero for the newborn infant to withstand drastic changes at birth — from the fetal circulatory pattern with liquid-filled lungs to air breathing with transitional circulatory adaption in a matter of a few minutes.
The newborn infant must exercise an effective neuronal drive and respiratory muscles to displace the liquid filling the airway system and to introduce sufficient air against the surface force in order to establish sufficient alveolar surface for gas exchange. At the same time, pulmonary blood vessels must dilate rapidly to increase pulmonary blood flow and to establish adequate regional alveolar ventilation/pulmonary perfusion (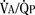 ) for sufficient pulmonary gas exchange. The neonatal adaptation of lung mechanics and respiratory control takes several weeks to complete. Beyond this immediate neonatal period, the infant’s lungs continue to mature at a rapid pace, and postnatal development of the lungs and the thorax surrounding the lungs continues well beyond the first year of life. Respiratory function in infants and toddlers, especially during the first several months of life, as with cardiovascular system and hepatic function, is both qualitatively and quantitatively different from that in older children and adults, and so is their responses to pharmacologic agents, especially anesthetics.
) for sufficient pulmonary gas exchange. The neonatal adaptation of lung mechanics and respiratory control takes several weeks to complete. Beyond this immediate neonatal period, the infant’s lungs continue to mature at a rapid pace, and postnatal development of the lungs and the thorax surrounding the lungs continues well beyond the first year of life. Respiratory function in infants and toddlers, especially during the first several months of life, as with cardiovascular system and hepatic function, is both qualitatively and quantitatively different from that in older children and adults, and so is their responses to pharmacologic agents, especially anesthetics.
Among many physiologic adaptations for the survival of humans at birth, cardio respiratory adaptation is by far the most crucial. The respiratory and circulatory systems must be developed sufficiently in utero for the newborn infant to withstand drastic changes at birth — from the fetal circulatory pattern with liquid-filled lungs to air breathing with transitional circulatory adaption in a matter of a few minutes.
The newborn infant must exercise an effective neuronal drive and respiratory muscles to displace the liquid filling the airway system and to introduce sufficient air against the surface force in order to establish sufficient alveolar surface for gas exchange. At the same time, pulmonary blood vessels must dilate rapidly to increase pulmonary blood flow and to establish adequate regional alveolar ventilation/pulmonary perfusion (
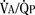 ) for sufficient pulmonary gas exchange. The neonatal adaptation of lung mechanics and respiratory control takes several weeks to complete. Beyond this immediate neonatal period, the infant’s lungs continue to mature at a rapid pace, and postnatal development of the lungs and the thorax surrounding the lungs continues well beyond the first year of life. Respiratory function in infants and toddlers, especially during the first several months of life, as with cardiovascular system and hepatic function, is both qualitatively and quantitatively different from that in older children and adults, and so is their responses to pharmacologic agents, especially anesthetics.
) for sufficient pulmonary gas exchange. The neonatal adaptation of lung mechanics and respiratory control takes several weeks to complete. Beyond this immediate neonatal period, the infant’s lungs continue to mature at a rapid pace, and postnatal development of the lungs and the thorax surrounding the lungs continues well beyond the first year of life. Respiratory function in infants and toddlers, especially during the first several months of life, as with cardiovascular system and hepatic function, is both qualitatively and quantitatively different from that in older children and adults, and so is their responses to pharmacologic agents, especially anesthetics.
This chapter reviews clinically relevant aspects of the development of the respiratory system and function in infants and children and their application to pediatric anesthesia. Such knowledge is indispensable for the proper care of infants and children before, during, and after general anesthesia and surgery, as well as for the care of those with respiratory insufficiency.
The respiratory system consists of the respiratory centers in the brainstem; the central and peripheral chemoreceptors; the phrenic, intercostal, hypoglossal (efferent), and vagal (afferent) nerves; the thorax (including the thoracic cage; the muscles of the chest, abdomen, and diaphragm); the upper (extrathoracic) and lower (intrathoracic) airways; the lungs; and the pulmonary vascular system. The principal function of the respiratory system is to maintain the oxygen and carbon dioxide (CO2) equilibrium in the body. The lungs also make an important contribution to the regulation of acid-base (pH) balance. The maintenance of body temperature (via loss of water through the lungs) is an additional but secondary function of the lungs. The lungs are also an important organ of metabolism.
Development
Development
Development of the human respiratory system
Prenatal Development of the Lungs
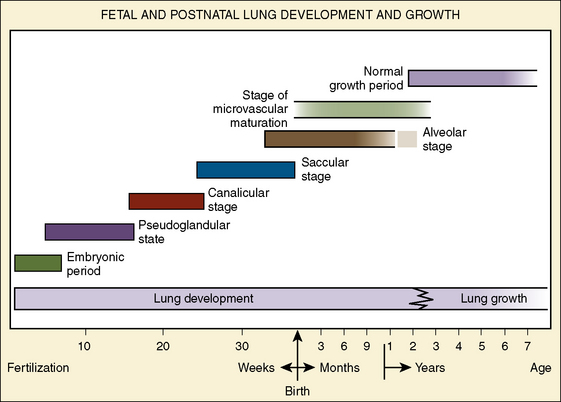
The morphologic development of the human lung is seen as early as several weeks into the embryonic period and continues well into the first decade of postnatal life and beyond (Fig. 3-1). The fetal lungs begin to form within the first several weeks of the embryonic period, when the fetus is merely 3 mm in length. A groove appears in the ventral aspect of the foregut, creating a small pouch. The outgrowth of the endodermal cavity, with a mass of surrounding mesenchymal tissue, projects into the pleuroperitoneal cavity and forms lung buds. The future alveolar membranes and mucous glands are derived from the endoderm, whereas the cartilage, muscle, elastic tissue, and lymph vessels originate from the mesenchymal elements surrounding the lung buds (Emery, 1969).

FIGURE 3-1 Stages of human lung development and their timing. Note the overlap between stages, particularly between the alveolar stage and the stage of microvascular maturation. Open-ended bars indicate uncertainty as to exact timing.
(From Zeltner TB, Burri PH: The postnatal development and growth of the human lung. II. morphology, Respir Physiol 67:269, 1987.)
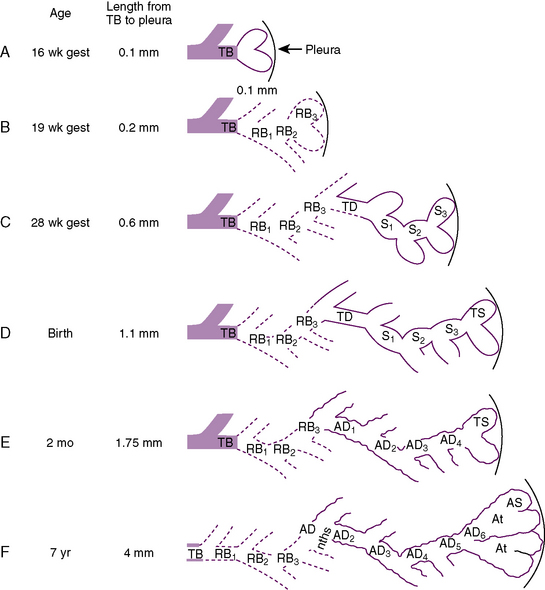
During the pseudoglandular period, which extends until 17 weeks’ gestation, the budding of the bronchi and lung growth rapidly take place, forming a loose mass of connective tissue. The morphologic development of the human lung is illustrated in Figure 3-2. By 16 weeks’ gestation, preacinar branching of the airways (down to the terminal bronchioli) is complete (Reid, 1967). A disturbance of the free expansion of the developing lung during this stage, as occurs with diaphragmatic hernia, results in hypoplasia of the airways and lung tissue (Areechon and Reid, 1963). During the canalicular period, in midgestation, the future respiratory bronchioli develop as the relative amount of connective tissue diminishes. Capillaries grow adjacent to the respiratory bronchioli, and the whole lung becomes more vascular (Emery, 1969).

FIGURE 3-2 Development of the acinus in human lungs at various ages. TB, Terminal bronchiole; RB, respiratory bronchiole; TD, transitional duct; S, saccule; TS, terminal saccule; AD, alveolar duct; At, atrium; AS, alveolar sac,
(From Hislop A, Reid L: Development of the acinus in the human lung, Thorax 29:90, 1974.)
At about 24 weeks’ gestation, the lung enters the terminal sac period, which is characterized by the appearance of clusters of terminal air sacs, termed saccules, with flattened epithelium (Hislop and Reid, 1974). These saccules are large and irregular with thick septa and have few capillaries in comparison with the adult alveoli (Boyden, 1969). At about 26 to 28 weeks’ gestation, proliferation of the capillary network surrounding the terminal air spaces becomes sufficient for pulmonary gas exchange (Potter, 1961). These morphologic developments may occur earlier in some premature infants (born at 24 to 25 weeks’ gestation) who have survived through neonatal intensive care. Starting at 28 weeks’ gestation, air space wall thickness decreases rapidly. From this period onward toward term, there is further lengthening of saccules with possible growth of additional generations of air spaces. Some mammalian species, such as the rat, have no mature alveoli at birth (Burri, 1974). In contrast, alveolar development from saccules begins in some human fetuses as early as 32 weeks’ gestation, but alveoli are not uniformly present until 36 weeks’ gestation (Langston et al., 1984). Most alveolar formation in humans takes place postnatally during the first 12 to 18 months of postnatal life, and development of respiratory bronchioles by transformation of preexisting terminal airways does not take place until after birth (Langston et al., 1984).
The fetal lung produces a large quantity of liquid, which expands the airways while the larynx is closed. This expansion produces the growth factor, such as human bombesin, and helps to stimulate lung growth and development (Sunday et al., 1988). The lung fluid is periodically expelled into the uterine cavity and contributes about one third of the total amniotic fluid. Prenatal ligation or occlusion of the trachea was tried in the 1990s with some success for the treatment of the fetus with congenital diaphragmatic hernia (Harrison et al., 1993). This treatment causes the expansion of the fetal airways and results in an accelerated growth of the otherwise hypoplastic lung.
The type II pneumocytes, which produce pulmonary surfactant that forms the alveolar lining layer, reduces surface tension and stabilizes air spaces after air breathing, appear at about 24 to 26 weeks’ gestation but occasionally as early as 20 weeks (Spear et al., 1969; Lauweryns, 1970). Idiopathic (or infantile) respiratory distress syndrome (IRDS), also known as hyaline membrane disease (HMD), which occurs in premature infants, is caused by the immaturity of the lungs with their insufficient pulmonary surfactant production and their inactivation by plasma proteins exudating onto the alveolar surface (see Surface Activity and Pulmonary Surfactant).
Experimental evidence from animals indicates that certain pharmacologic agents such as cortisol and thyroxin administered to the mother or directly to the fetus accelerate the maturation of the lungs, resulting in the early appearance of type II pneumocytes and surfactant (deLemos et al., 1970; Motoyama et al., 1971; Wu et al., 1973; Smith and Bogues, 1982; Rooney, 1985). Liggins and Howie (1972) reported accelerated maturation of human fetal lungs after the administration of corticosteroids to mothers 24 to 48 hours before the delivery of premature babies. Despite initial concern that steroids might potentially be toxic to other organs of the fetus, particularly to the development of the central nervous system, prenatal glucocorticoid therapy has been used widely since the 1980s to induce lung maturation and surfactant synthesis in mothers at risk of premature delivery (Avery, 1984; Avery et al., 1986).
Neonatal respiratory adaptation
Respiratory rhythmogenesis occurs in the fetus long before partition. The clamping of the umbilical cord and increasing arterial oxygen tensions and relative hyperoxia with air breathing (but not transient hypoxia) initiate and maintain rhythmic breathing at birth.
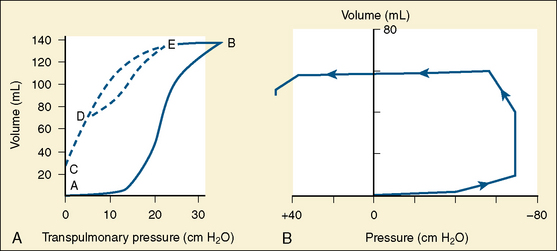
To introduce air into the fluid-filled lungs at birth, the newborn infant must overcome large surface force with the first few breaths. Usually a negative pressure of 30 cm H2O is necessary to introduce air into the fluid-filled lungs. In some normal full-term infants, even with sufficient surfactant, a force of as much as −70 cm H2O or more must be exerted to overcome the surface force (Fig. 3-3) (Karlberg et al., 1962). Usually fluid is rapidly expelled via the upper airways. The residual fluid leaves the lungs through the pulmonary capillaries and lymphatic channels over the first few days of life, and changes in compliance parallel this time course. All changes are delayed in the premature infant.

FIGURE 3-3 A, Typical pressure-volume curve of expansion of a gas-free lung. A-B, initial expansion. In the example, approximately 30 cm H2O pressure will be necessary to overcome surface forces. C, Deflation to zero pressure with gas trapping. D-E, Subsequent breaths with a further increase in FRC (from C to D). B, Pressure-volume relationships during the first breath of a newborn weighing 4.3 kg. Here, 60 to 70 cm H2O negative pressure was necessary to overcome the surface forces.
(From Karlberg P et al.: Respiratory studies in newborn infants. II. Pulmonary ventilation and mechanics of breathing in the first minutes of life, including the onset of respiration, Acta Paediatr Scand 51:121, 1962.)
As the lungs expand with air, pulmonary vascular resistance decreases dramatically and pulmonary blood flow increases markedly, thus allowing gas exchange between alveolar air and pulmonary capillaries to occur. Changes in Po2, Pco2 (P stands for partial pressure), and pH are largely responsible for the dramatic decrease in pulmonary vascular resistance (Cook et al., 1963). The resultant large increases in pulmonary blood flow and the increase in left atrial pressure with a decrease in right atrial pressure reverse the pressure gradient across the atria and close (initially functionally and eventually anatomically) the foramen ovale, a right-to-left one-way valve. With these adjustments, the cardiopulmonary system approaches adult levels of ventilation/perfusion (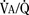 balance within a few days (Nelson et al., 1962, 1963). The process of expansion of the lungs during the first few hours of life and the resultant circulatory adaptation for establishing pulmonary gas exchange are greatly influenced by the adequacy of pulmonary surfactant. It should be remembered that these changes are delayed in immature newborns.
balance within a few days (Nelson et al., 1962, 1963). The process of expansion of the lungs during the first few hours of life and the resultant circulatory adaptation for establishing pulmonary gas exchange are greatly influenced by the adequacy of pulmonary surfactant. It should be remembered that these changes are delayed in immature newborns.
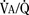 balance within a few days (Nelson et al., 1962, 1963). The process of expansion of the lungs during the first few hours of life and the resultant circulatory adaptation for establishing pulmonary gas exchange are greatly influenced by the adequacy of pulmonary surfactant. It should be remembered that these changes are delayed in immature newborns.
balance within a few days (Nelson et al., 1962, 1963). The process of expansion of the lungs during the first few hours of life and the resultant circulatory adaptation for establishing pulmonary gas exchange are greatly influenced by the adequacy of pulmonary surfactant. It should be remembered that these changes are delayed in immature newborns.Postnatal Development of the Lungs and Thorax
The development and growth of the lungs and surrounding thorax continue with amazing speed during the first year of life. Although the formation of the airway system all the way to the terminal bronchioles is complete by 16 weeks’ gestation, alveolar formation begins only at about 36 weeks’ gestation. At birth, the number of terminal air sacs (most of which are saccules) is between 20 and 50 million, only one tenth that of fully grown lungs of the child. Most postnatal development of alveoli from primitive saccules occurs during the first year and is essentially completed by 18 months of age (Langston et al., 1984). The morphologic and physiologic development of the lungs, however, continues throughout the first decade of life (Mansell et al., 1972).
During the early postnatal period, the lung volume of infants is disproportionately small in relation to body size. In addition, because of higher metabolic rates in infants (oxygen consumption per unit body weight is twice as high as that of adults), the ventilatory requirement per unit of lung volume in infants is markedly increased. Infants, therefore, have much less reserve of lung volume and surface area for gas exchange. This is the primary reason why infants and young children become rapidly desaturated with hypoventilation or apnea of relatively short duration.
In the neonate, static (elastic) recoil pressure of the lungs is very low (i.e., compliance, normalized for volume, is unusually high), which is not dissimilar to that of geriatric or emphysematous lungs, because the elastic fibers do not develop until the postnatal period (whereas elastic fibers in geriatric lungs are brittle and not functional) (Mansel et al., 1972; Fagan, 1976; Bryan and Wohl, 1986). In addition, the elastic recoil pressure of the infant’s thorax (chest wall) is extremely low because of its compliant cartilaginous rib cage with poorly developed thoracic muscle mass, which does not add rigidity. These unique characteristics make infants more prone to lung collapse, especially under general anesthesia when inspiratory muscles are markedly relaxed (see maintenance of FRC below). Throughout infancy and childhood, static recoil pressure of the lungs and thorax steadily increases (compliance, normalized for volume, decreases) toward normal values for young adults (Zapletal et al., 1971; Motoyama, 1977).
The actual size of the airway from the larynx to the bronchioles in infants and children, of course, is much smaller than in adolescents and adults, and flow resistance in absolute terms is extremely high. When normalized for lung volume or body size, however, infants’ airway size is relatively much larger; airway resistance is much lower than in adults (Polgar, 1967; Motoyama, 1977; Stocks and Godfrey, 1977). Infants and toddlers, however, are more prone to severe obstruction of the upper and lower airways because their absolute (not relative) airway diameters are much smaller than those in adults. As a consequence, relatively mild airway inflammation, edema, or secretions can lead to far greater degrees of airway obstruction than in adults (e.g., as with subglottic croup [laryngotracheobronchitis] or acute supraglottitis [epiglottitis]).
Further description on the development of the lungs and thorax and their effects on lung function, especially under general anesthesia, are described later in the chapter. Perinatal and postnatal adaptations of respiratory control are included in the following section on the control of breathing.
NEXT》》CLICK HERE
NEXT》》CLICK HERE

No comments:
Post a Comment