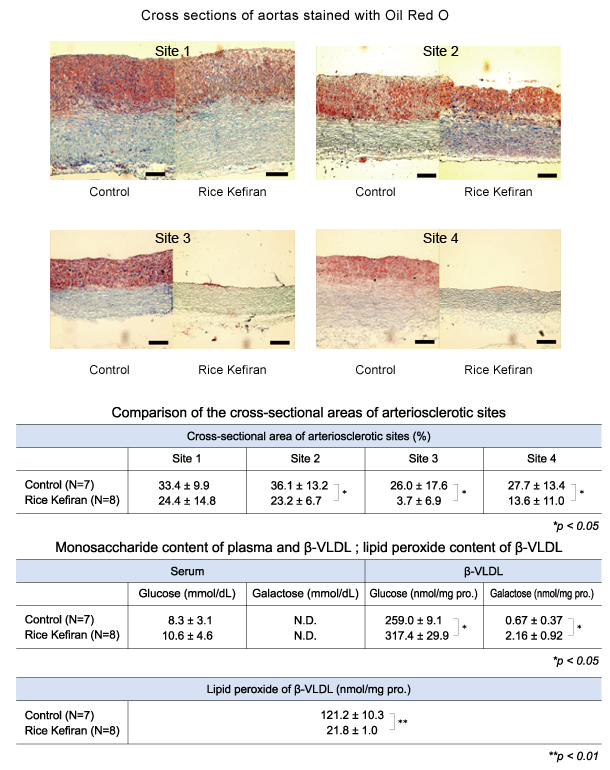
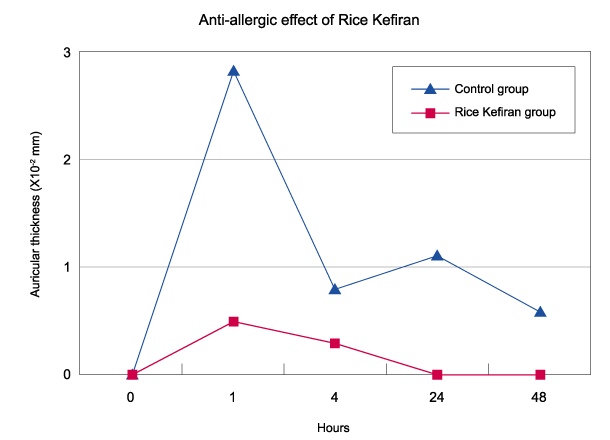
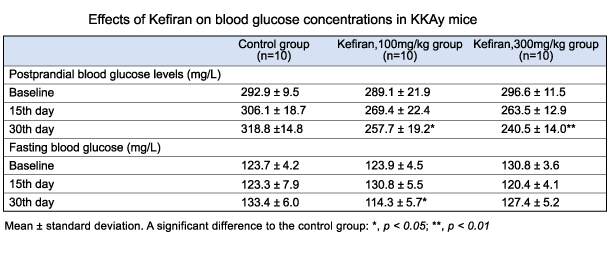
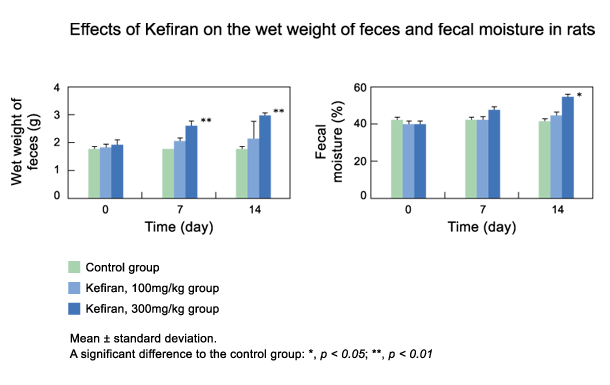
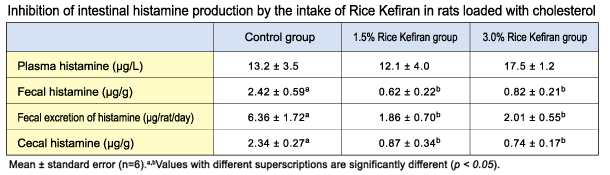
1 - superficial temporal
2 - occipital
3 - maxillary
4 - vertebral
5 - facial
6 - lingual
7 - common carotid
8 - subclavian
9 - arch of aorta
10 - superior vena cava
11 - heart
12 - superior mesenteric
13 - inferior vena cava
14 - liver
15 - renal
16 - kidneys
17 - spleen
18 - radial
19 - ulnar
20 - common iliac
21 - hypogastric
22 - external iliac
23 - femoral
24 - saphenous right
25 - popliteal
26 - post, tibial
27 - anterior tibial
28 - saphenous left
29 - dorsalis pedis
30 - dorsalis venous
31 - arcuate

VITAL NUTRIENTS
Calcium regulates and assists the smooth functioning of the heartbeat. Magnesium and calcium nourish the circulatory system.
Phosphorus is essential for proper blood circulation, regulation of blood pressure levels and heart muscle contractions.
Potassium is essential for the heart muscle, proper blood circulation, heart muscle contractions and a regular heartbeat.
Iron is required to carry oxygen.
Magnesium assists nerve impulses for the heart.
Silicon assists blood circulation.
Iodine protects against heart palpitations.
Sodium maintain blood pressure.
Vanadium regulates blood circulation.
Vitamin E promotes the ability of red blood cells to carry oxygen, it assists the heart muscles to use oxygen more efficiently and protects against blood clots.
Vitamin C and P promote strong blood capillaries. Vitamin P is a rarely used collective term for a plant classification known as flavonoids, or bioflavonoids -- the terms more commonly used. What foods are high in vitamin P?
Flavonols: Vegetables, fruits, onions, grape seed, pine bark.
Flavanones: Citrus fruit, licorice.
Flavones: Celery, parsley, red peppers, chamomile, mint, ginkgo biloba.
Flavanolols: Milk thistle, propolis.
Flavanols: Tea, cocoa, chocolate, azaleas, grape seed.
Vitamin B15 protects against hardened arteries. In Europe, vitamin B15 has been used to treat premature aging because of both its circulatory stimulus and its antioxidant effects. It helps protect the body from pollutants, especially carbon monoxide. Pangamic acid (and possibly DMG) offers support for anyone living in a large polluted city or under high-stress. Food sources of pangamic acid include beef blood, whole grains such as brown rice, brewer's yeast, pumpkin and sunflower seeds and apricot seeds.
(to study heart I click here)
CIRCULATORY SYSTEM
HEART The human heart pumps blood at 72 beats per minute (60seconds) for as adult, 90 beats per minute for a child, and 110 beats per minute for an infant.
LIVER The human liver removes toxins from the bloodstream at a rate of at least 1.5 litres every minute (60 seconds).
KIDNEYS The human kidneys are a part of filters with remarkable ability to regulate the amount of blood salt and acid levels. The kidneys filter blood at the rate of 1.3 litres per minute (60 seconds) with millions of tiny tubes called nephrons.
SPLEEN The spleen destroys old red blood cells and blood platelets and provide lymphocytes for new blood.
ARTERIES The blood leaves the heart via the pulmonary artery and enters the lungs where oxygen is added. The combined network of arteries and veins within the circulatory system measure approx. 96,000 kilometres.
VEINS Veins carry oxygenated blood throughout the human body.
BENEFICIAL NATURAL FOODS
1. tahini, cheese, almonds, hazel nuts, sunflower seeds, dried apricots, spinach.
2. pepitas, sunflower seeds, tahini, Brazil nuts, cashews, walnuts, almonds, lecithin.
3. dried apricots, dried fruits, fresh wheatgrass, legumes, almonds, sunflower seeds.
4. pepitas, tahini, parsley.
5. Brazil nuts, tahini, almonds, cashews, lettuce, spinach, strawberry.
6. kelp, watermelon, capsicum.
7. celery, olives, spinach, cheese.
8. fresh fruits, tuna, kelp, seafood.
9. sunflower seeds, tahini, almonds, hazel nuts, fresh wheatgerm, peanut, apples, lettuce, walnuts, Brazil nuts.
10. guava, capsicum, citrus fruits.
11. pepitas, tahini, oats, wheatgerm.
Tahini is made from sesame seeds that are soaked in water and then crushed to separate the bran from the kernels. The crushed seeds are soaked in salt water, causing the bran to sink. The floating kernels are skimmed off the surface, toasted, and ground to produce an oily paste.
Pepitas are pumpkin seeds. They're eaten as a snack or added to salads for a little crunch. You can find them roasted, raw or salted, in the shell or hulled. Pumpkin seeds contain healthy fats as well as protein, fiber, potassium, iron and zinc.
4 Alkaline Minerals for Ph Balance in Your Body.
There are many acidic foods that may erode your alkaline health one meal at a time. A proven way to counter yourself and your family from the side effects of acidic foods is to follow the 80/20 rule of the alkaline diet. Simply put, for every 20 percent of acid-forming foods you consume you should add 80 percent of alkalizing foods to your diet. That way you’ll keep your Ph levels and body happy.
(Note: Not all acidic foods are acid-forming in your body. The lemon, for example, is an acidic fruit. However, when consumed it is alkaline forming in your body, because it is loaded with calcium, magnesium and potassium.)
Supplement your diet with alkaline minerals.
The main alkalizing minerals are calcium, magnesium, sodium and potassium. These minerals complement each other in your body. For example, calcium is needed to contract a muscle and magnesium is needed to relax it. So, you need both.
Calcium and Magnesium
Without calcium our brain, muscles, and nerves would not work properly.The best source of calcium in food is green leafy vegetables. Some fresh vegetables, such as broccoli, cabbage, and spinach, contain calcium. So do almonds, sesame seeds, and soft-boned fish, like sardines and salmon.
The body requires magnesium to maintain energy and vitality. When magnesium is deficient in the body, the body forms lactic acid. Low levels of magnesium are associated with migraine headaches, nervousness, insomnia, and heart palpitations. So, make sure you get adequate amounts of magnesium.
Eat Potassium-rich Foods
What about potassium? Potassium is essential for muscular activity, particularly so for that tireless muscle, the heart.If you do not get enough potassium, you may suffer from weakness of muscles, poor reflexes, back pain, headaches, constipation or sleeplessness. You may also find yourself subject to apathy, listlessness, depression or mental confusion. Extreme lack of potassium can increase the risk of a heart attack.
What’s more, at a cellular level your cells maintain a balance of potassium inside and sodium outside, but this pumping of potassium and sodium requires magnesium.
The calcium concentration in cells is controlled by sodium. You see all four of these minerals work together in the body. Most importantly, these minerals are critical in keeping your body, organs and tissue Ph balanced.
Problems arise in the body when one or more of the minerals are deficient or when the minerals are out of balance with each other.
Things like stress, age, pollution, diet and many other factors deplete your body of these critical minerals and make your body less efficient at removing waste and acid deposits from your blood, tissues and organs. And so your body becomes dangerously acidic!
Conclusion…
STEP 1 – you should supplement your diet with these critical minerals to restore Ph balance to your body.
STEP 2 – you must eat more alkalizing foods to help your body eliminate waste effectively and resort its critical Ph balance.
STEP 3 – Drink ionized alkaline water that has these minerals in them.
It makes absolute sense to assist your body in eliminating wastes and toxins by eating liberal amounts of alkaline-rich foods. The Alkaline Cookbook provides a simple alkaline diet eating plan to help your cleanse and nourish your body, mind and soul using alkalizing foods. You see, your body will thrive on alkalizing foods! As a result, you will have more energy, increased stamina, less stress in your life and your body will be better equipped to ward off disease, viruses and infections.
It makes absolute sense to assist your body in eliminating wastes and toxins by eating liberal amounts of alkaline-rich foods. The Alkaline Cookbook provides a simple alkaline diet eating plan to help your cleanse and nourish your body, mind and soul using alkalizing foods. You see, your body will thrive on alkalizing foods! As a result, you will have more energy, increased stamina, less stress in your life and your body will be better equipped to ward off disease, viruses and infections.
The Alkaline Cookbook easy-to-make recipes make you feel FULL and satisfied with every meal. Yes, you will feel fantastic with every meal!