The condition of the body is maintained at a constant level by modulating body temperature and blood pressure, to cope with changes in the environment inside and outside the body. This mechanism is called homeostasis. In homeostasis, the innate immune system plays an important role: it eliminates foreign substances (non-self) such as pathogens, viruses and cancer cells. However, it is known that immune function deteriorates with aging, and is impaired or stimulated by a poor lifestyle such as smoking, insufficient physical exertion or an unbalanced diet, and by adverse environmental changes and pollution. Deterioration of immune function causes or aggravates infection and malignant tumors, while excessive elevation of the immune system may cause things such as hay fever, atopic dermatitis or chronic inflammation. Studies in recent years have clarified that fiber and indigestive components in food are deeply involved in the maintenance of homeostasis and immune responsiveness.

BioBran is a functional food manufactured using a water-soluble rice bran dietary fiber component (hemicellulose B). Collaborative research with Prof. Mamdooh Ghoneum Ph.D. at the University of California, Los Angeles, USA (UCLA/Drew University of Medicine) on rice bran dietary fiber components has led to the discovery and establishment of processing and manufacturing methods of a food that optimally modulates the innate immune function of the body. It was subsequently demonstrated that BioBran has not only an immunostimulatory effect but also an immunomodulating effect. Extensive efforts to maximize its stability and taste have resulted in BioBran rice bran arabinoxylan. Currently, BioBran is widely used not only in Japan but also in more than 50 countries worldwide, and has an excellent reputation.
Patent No.5358219.
Daiwa Pharmaceutical Co., Ltd. has focused on a dietary fiber thought to have the potential to impact the immune response and developed a multifunctional food closely related to the Japanese foodstuff of ancient times, named BioBran (Rice Bran Arabinoxylan Compound).
Manufacturing Process
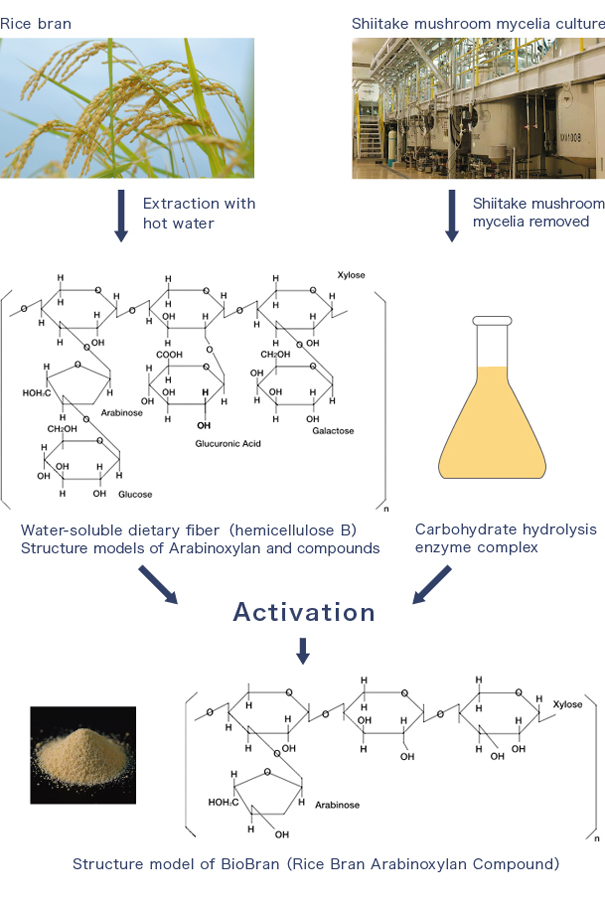

Characteristics
BioBran is a functional food produced by water-soluble dietary fiber (hemicellulose B) with an enzyme derived from shiitake mushrooms.
Suggested daily dose
1-3g/day
Property
Easy soluble in water and heat-stable
Mechanism of Action
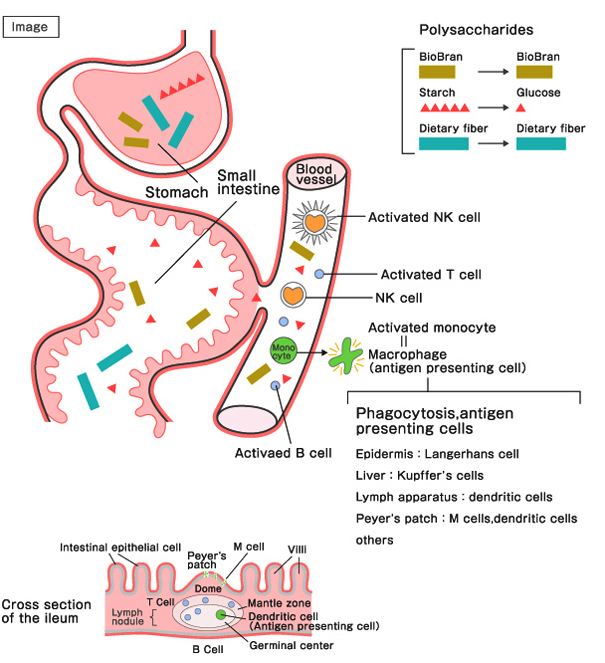
Starch, dietary fiber, and BioBran are classified as polysaccharides.
Starch is digested by saliva, pancreatic juice, and intestinal juice, and is absorbed in the small intestine as glucose. Dietary fiber is not digested, and is excreted unchanged. BioBran is known to be partly absorbed directly in the digestive tract, entering the blood stream.It activates the Natural Killer Cells, T cells, B cells, and macrophages, either directly in the blood, or indirectly via the Peyer's patch of the ileum, BioBran plays an important role in immunomodulation (immunostimulation, anti-inflammation, anti-allergy, antioxidation processes). Overall, it is considered to enhance the body's natural healing abilities, alleviate the adverse drug reactions of chemotherapeutic agents, and improve quality of life.


Usefulness as a functional food
1. Immunomodulatory action
(1) Immunostimulating action
▶Response of human NK cell activation to dose of BioBran
▶NK cell activation in cancer patients following BioBran intake
▶Stimulation of lymphocyte transformation
▶Promotion of differentiation of dendritic cells
▶Anticancer effect of BioBran
(2) Anti-inflammatory action
▶Influence on liver damage
2. Combination therapy with a chemotherapeutic agent
▶BioBran in combination with a chemotherapeutic agent
3. Improvement of quality of life
▶Effect of BioBran on survival and quality of life improvement in patients with progressive cancer
Safety
Single dose ~ LD50>36g/kg.
Repeated dose ~ NOAEL>200mg/kg/day.
Mutagenicity (Ames test) ~ Negative.
▶Response of human NK cell activation to dose of BioBran
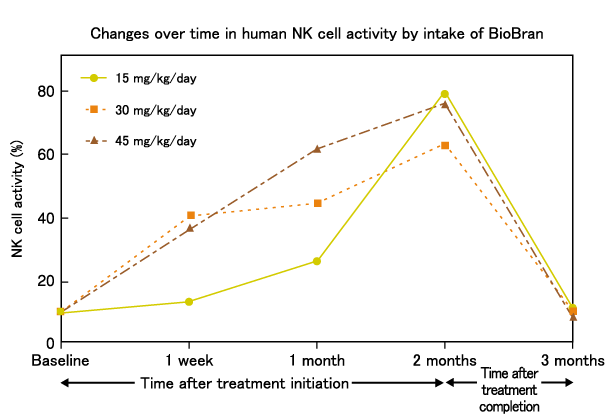
Twenty-four healthy human volunteers were divided into three groups of 8, and assigned to ingest BioBran at 15mg/kg body weight (approximately equal to 1g), 30mg/kg body weight (approximately equal to 2g), and 45mg/kg body weight (approximately equal to 3g) every day for a period of two months to measure the effects on NK cell activity.
The activity in the 15mg/kg group was almost unchanged after 1 week but increased to twice the baseline activity after 1 month. The activity in the 30mg/kg group increased to about 3 times the baseline value after 1 week and then gradually increased to 5 times the baseline value by 2 months. The 45mg/kg group showed a pattern of increase similar to that in the 30mg/kg group, but the rate and degree of increase were greater than that of the 30mg/kg group. Following cessation of treatment, the NK cell activity levels gradually returned to baseline in each group.
Response of human NK cell activation to dose of BioBran :
Ghoneum M.,“Enhancement of human natural killer cell activity by modified Arabinoxylan from rice bran (MGN-3)”, INT. J. IMMUNOTHERAPY XIV (2) 89-99 (1998)
▶NK cell activation in cancer patients following BioBran intake
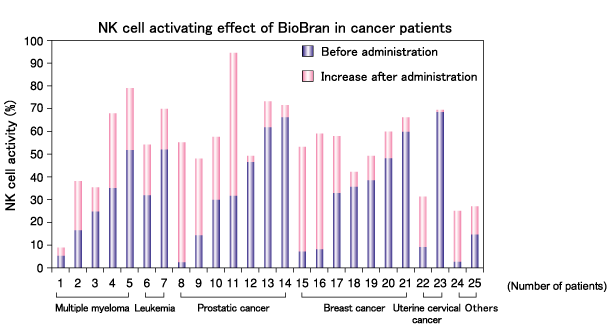
NK cell activity was compared before and after 6 months of BioBran intake in 25 cancer patients with progressive disease, who received chemotherapy, surgery, and/or hormone therapy. Although a large difference in the baseline activity was observed between patients, all patients demonstrated an increase in NK cell activity after BioBran intake.
NK cell activation in cancer patients following BioBran intake :
Ghoneum M. and G. Namatalla, 87th Annual Meeting of the American Association for Cancer Research, 1996.
▶Stimulation of lymphocyte transformation
The proliferation of T-cell and B-cell lymphocytes was compared before and after BioBran intake. The proliferation of both T-cells and B-cells increased in the 3 patients following BioBran intake.
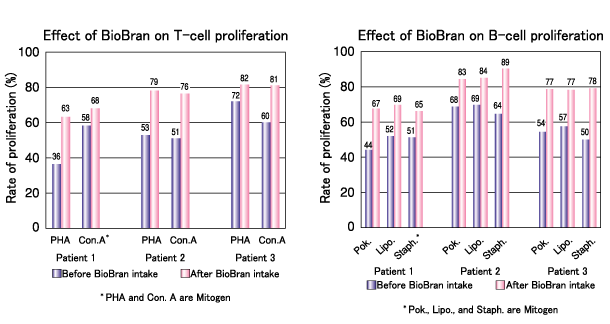
Stimulation of lymphocyte transformation:
Ghoneum M., 11th International AIDS Conference in Vancouver, 1996.
▶Promotion of differentiation of dendritic cells
Monocytes were isolated from the peripheral blood of healthy subjects and cultured in the presence of GM-CSF and IL-4 for 6 days, to prepare immature dendritic cells (iDC). On the 7th day, BioBran was added at various concentrations to the immature dendritic cells. The immature dendritic cells were incubated for 2 days and their maturation to dendritic cells was observed. The same operation was performed using two kinds of culture medium which have the effect of promoting the development of iDC to mature dendritic cells, designated mat DC1, and matDC2, respectively. In the immature dendritic cells (iDC), BioBran suppressed the expression of the monocyte marker CD14 and increased the expression of the dendritic cell marker CD83, both in a dose dependent manner. This result appears to show that BIoBran promotes the differentiation of dendritic cells.
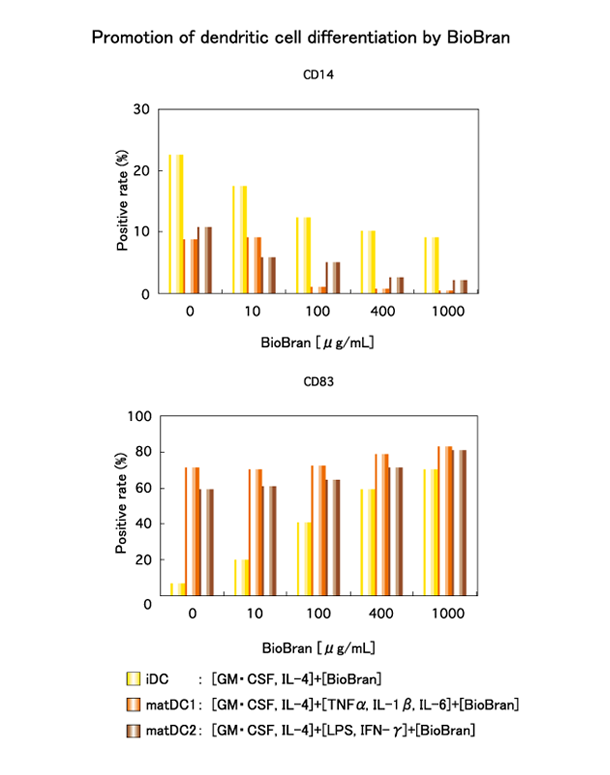
Promotion of differentiation of dendritic cells :
Cholujova D., et al, “BioBran-augmented maturation of human monocyte-derived dendritic cells”, NEOPLASMA, 56, 2, 2009.
▶Anticancer effect of BioBran
Female Swiss albino mice were inoculated with 2.5 x 106 Ehrlich ascites carcinoma (EAC) cells into the right femoral region, and the tumor size determined on consecutive days, starting on the 8th day after inoculation until the 35th day. The control group received phosphate buffered saline (PBS), and the BioBran group received 40 mg/kg body weight of BioBran intraperitoneally three times a week for a period of 3 weeks, starting on the 8th day after the inoculation of EAC. After treatment, the tumor size was determined. The results showed a significantly greater suppression of tumor growth in the BioBran group compared to the control (PBS) group, starting on the 14th day after inoculation of EAC. The photographs of tumor on the 35th day also showed a tumor regression in the BioBran group.
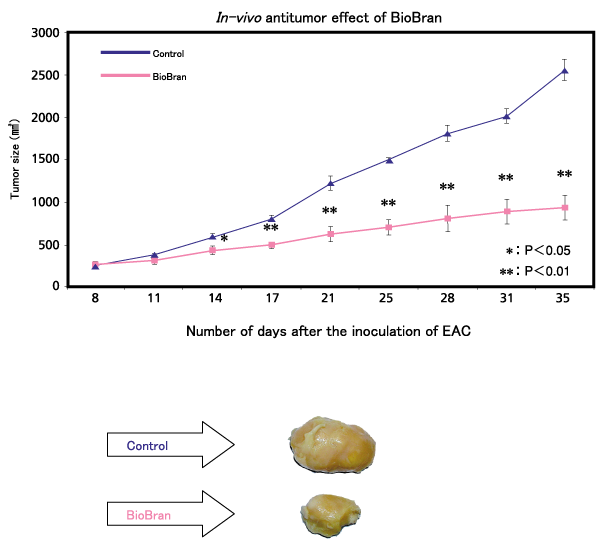
バイオブランのin vivoにおける抗腫瘍効果
Anticancer effect of BioBran :
Ghoneum M., et al, “In vivo Tumor Inhibitory Effects of Nutritional Rice Bran Supplement MGN-3/Biobran on Ehrlich Carcinoma-Bearing Mice”, Nutrition and Cancer, 2008.
▶Influence on liver damage
Male Wistar rats (5 rats/group) were given intraperitoneal administration of 800 mg/kg D-galactosamine to induce liver disorder. Serum GOT and GPT were determined 24 hours after the administration of D-galactosamine, as induces of liver damage. One hour before administration of D-galactosamine, 20, 40, or 80 mg/kg of BioBran was administered intraperitoneally. The serum GOT and GPT in the control group without BioBran at 24 hours after the administration of D-galactosamine were 1410 IU/L and 445 IU/L, respectively. Compared to these values, serum GOT and GPT in the groups treated with BioBran were significantly lower at all doses.
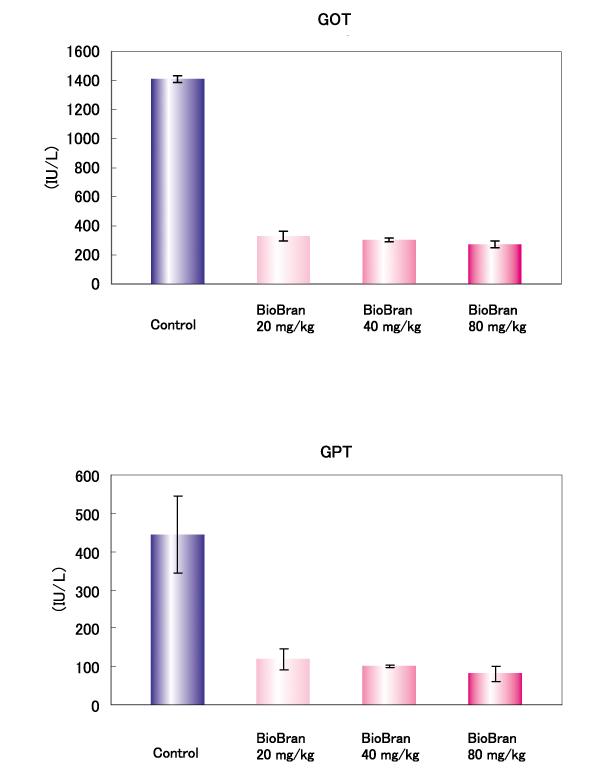
Influence on liver damage :
Sanada H. and Y. Egashira, 7th meeting of the Japanese Association for Dietary Fiber Research, Tokyo, 2002.
▶BioBran in combination with a chemotherapeutic agent
Human breast cancer cells (MCF-7) (1 x 104 cells) were seeded into the wells of a plate and incubated for 3 days in the presence of differing concentrations of BioBran, and the chemotherapeutic agent daunorubicin (DNR). Viable cells were counted by the MTT method, and the concentration of DNR which reduced the number of viable cancer cells by 50% (IC50) was determined. DNR inhibited the survival of MCF-7 cells in a concentration-dependent manner, with the IC50 being 1μM. When MCF-7 cells were cultured in the presence of BioBran and DNR, the IC50 of DNR for MCF-7 was reduced significantly (IC50: 0.2μM).
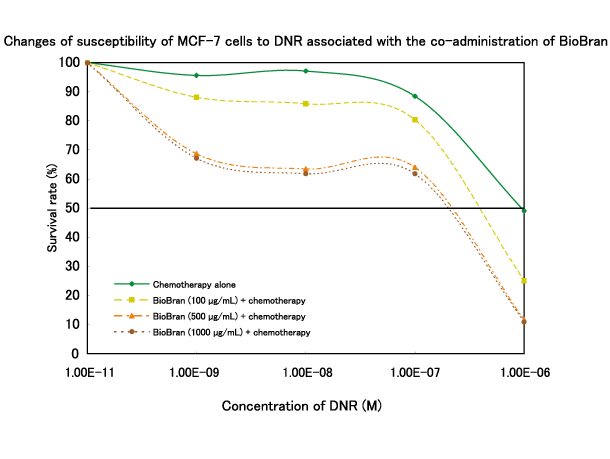
BioBran in combination with a chemotherapeutic agent :
Ghoneum M. and S. Gollapudi, “MGN-3/Biobran, modified arabinoxylan from rice bran, sensitizes human breast cancer cells to chemotherapeutic agent, daunorubicin”, Cancer Detection and Prevention, 2008.
▶Effect of BioBran on survival and quality of life improvement in patients with progressive cancer
Two hundred and five patients with malignant tumors who were receiving treatment with alternative therapy and chemotherapy with mild adverse drug reactions were allocated into two groups. One of the groups (the control group) received conventional alternative therapy and chemotherapy, and the other group (BioBran group) was given 1 g of BioBran three times a day after each meal for 18 months, in addition to receiving the same therapy as the control group. The correlation between natural killer (NK) cell activity determined during the observation period and survival rate was studied.
The patients’ quality of life was assessed on a scale of 0 to 4 with regard to “pain”, “fatigue”, and “nausea” and 0 to 3 with regard to “appetite” at the start of, and during, the observation period. Of the 205 patients participating in the study, 53 patients of the control group could not continue the conventional alternative therapy and dropped out, and therefore 152 patients (56 patients in the control group and 96 in the BioBran group) were included in the analysis. The survival rates at the end of the observation period in the control group and the BioBran group were 35.8% and 54.2%, respectively. This represents a 50% higher survival rate in the BioBran group compared to the control group. It was also shown that higher NK cell activity was associated with a higher survival rate (Table 1). It was confirmed that the patients’ quality of life after the study had improved compared to the quality of life at the start of the study in both the control group and BioBran group; a notable improvement in appetite was observed in the BioBran group (Table 2).
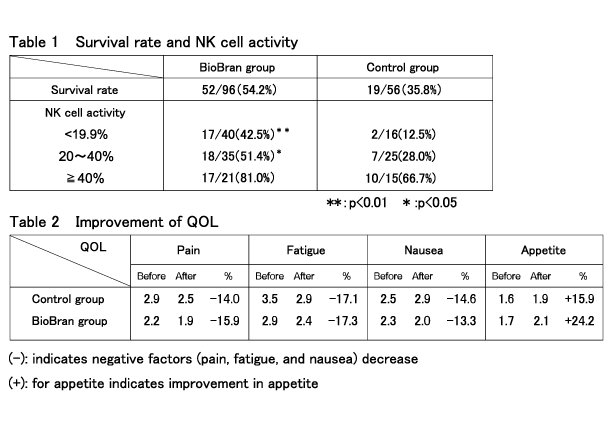
Effect of BioBran on survival and quality of life improvement in patients with progressive cancer :
Takahara K., et al, “The Life Prolongation and QOL Improvement Effect of Rice Bran Arabinoxylan Derivative (MGN-3, BioBran) for Progressive Cancer”, Clinical Pharmacology and Therapy, 2004

BioBran is a functional food manufactured using a water-soluble rice bran dietary fiber component (hemicellulose B). Collaborative research with Prof. Mamdooh Ghoneum Ph.D. at the University of California, Los Angeles, USA (UCLA/Drew University of Medicine) on rice bran dietary fiber components has led to the discovery and establishment of processing and manufacturing methods of a food that optimally modulates the innate immune function of the body. It was subsequently demonstrated that BioBran has not only an immunostimulatory effect but also an immunomodulating effect. Extensive efforts to maximize its stability and taste have resulted in BioBran rice bran arabinoxylan. Currently, BioBran is widely used not only in Japan but also in more than 50 countries worldwide, and has an excellent reputation.
Patent No.5358219.
Daiwa Pharmaceutical Co., Ltd. has focused on a dietary fiber thought to have the potential to impact the immune response and developed a multifunctional food closely related to the Japanese foodstuff of ancient times, named BioBran (Rice Bran Arabinoxylan Compound).
Manufacturing Process


Characteristics
BioBran is a functional food produced by water-soluble dietary fiber (hemicellulose B) with an enzyme derived from shiitake mushrooms.
Suggested daily dose
1-3g/day
Property
Easy soluble in water and heat-stable
Mechanism of Action
Starch, dietary fiber, and BioBran are classified as polysaccharides.
Starch is digested by saliva, pancreatic juice, and intestinal juice, and is absorbed in the small intestine as glucose. Dietary fiber is not digested, and is excreted unchanged. BioBran is known to be partly absorbed directly in the digestive tract, entering the blood stream.It activates the Natural Killer Cells, T cells, B cells, and macrophages, either directly in the blood, or indirectly via the Peyer's patch of the ileum, BioBran plays an important role in immunomodulation (immunostimulation, anti-inflammation, anti-allergy, antioxidation processes). Overall, it is considered to enhance the body's natural healing abilities, alleviate the adverse drug reactions of chemotherapeutic agents, and improve quality of life.



Usefulness as a functional food
1. Immunomodulatory action
(1) Immunostimulating action
▶Response of human NK cell activation to dose of BioBran
▶NK cell activation in cancer patients following BioBran intake
▶Stimulation of lymphocyte transformation
▶Promotion of differentiation of dendritic cells
▶Anticancer effect of BioBran
(2) Anti-inflammatory action
▶Influence on liver damage
2. Combination therapy with a chemotherapeutic agent
▶BioBran in combination with a chemotherapeutic agent
3. Improvement of quality of life
▶Effect of BioBran on survival and quality of life improvement in patients with progressive cancer
Safety
Single dose ~ LD50>36g/kg.
Repeated dose ~ NOAEL>200mg/kg/day.
Mutagenicity (Ames test) ~ Negative.
▶Response of human NK cell activation to dose of BioBran
Twenty-four healthy human volunteers were divided into three groups of 8, and assigned to ingest BioBran at 15mg/kg body weight (approximately equal to 1g), 30mg/kg body weight (approximately equal to 2g), and 45mg/kg body weight (approximately equal to 3g) every day for a period of two months to measure the effects on NK cell activity.
The activity in the 15mg/kg group was almost unchanged after 1 week but increased to twice the baseline activity after 1 month. The activity in the 30mg/kg group increased to about 3 times the baseline value after 1 week and then gradually increased to 5 times the baseline value by 2 months. The 45mg/kg group showed a pattern of increase similar to that in the 30mg/kg group, but the rate and degree of increase were greater than that of the 30mg/kg group. Following cessation of treatment, the NK cell activity levels gradually returned to baseline in each group.

Response of human NK cell activation to dose of BioBran :
Ghoneum M.,“Enhancement of human natural killer cell activity by modified Arabinoxylan from rice bran (MGN-3)”, INT. J. IMMUNOTHERAPY XIV (2) 89-99 (1998)
▶NK cell activation in cancer patients following BioBran intake
NK cell activity was compared before and after 6 months of BioBran intake in 25 cancer patients with progressive disease, who received chemotherapy, surgery, and/or hormone therapy. Although a large difference in the baseline activity was observed between patients, all patients demonstrated an increase in NK cell activity after BioBran intake.

NK cell activation in cancer patients following BioBran intake :
Ghoneum M. and G. Namatalla, 87th Annual Meeting of the American Association for Cancer Research, 1996.
▶Stimulation of lymphocyte transformation
The proliferation of T-cell and B-cell lymphocytes was compared before and after BioBran intake. The proliferation of both T-cells and B-cells increased in the 3 patients following BioBran intake.

Stimulation of lymphocyte transformation:
Ghoneum M., 11th International AIDS Conference in Vancouver, 1996.
▶Promotion of differentiation of dendritic cells
Monocytes were isolated from the peripheral blood of healthy subjects and cultured in the presence of GM-CSF and IL-4 for 6 days, to prepare immature dendritic cells (iDC). On the 7th day, BioBran was added at various concentrations to the immature dendritic cells. The immature dendritic cells were incubated for 2 days and their maturation to dendritic cells was observed. The same operation was performed using two kinds of culture medium which have the effect of promoting the development of iDC to mature dendritic cells, designated mat DC1, and matDC2, respectively. In the immature dendritic cells (iDC), BioBran suppressed the expression of the monocyte marker CD14 and increased the expression of the dendritic cell marker CD83, both in a dose dependent manner. This result appears to show that BIoBran promotes the differentiation of dendritic cells.

Promotion of differentiation of dendritic cells :
Cholujova D., et al, “BioBran-augmented maturation of human monocyte-derived dendritic cells”, NEOPLASMA, 56, 2, 2009.
▶Anticancer effect of BioBran
Female Swiss albino mice were inoculated with 2.5 x 106 Ehrlich ascites carcinoma (EAC) cells into the right femoral region, and the tumor size determined on consecutive days, starting on the 8th day after inoculation until the 35th day. The control group received phosphate buffered saline (PBS), and the BioBran group received 40 mg/kg body weight of BioBran intraperitoneally three times a week for a period of 3 weeks, starting on the 8th day after the inoculation of EAC. After treatment, the tumor size was determined. The results showed a significantly greater suppression of tumor growth in the BioBran group compared to the control (PBS) group, starting on the 14th day after inoculation of EAC. The photographs of tumor on the 35th day also showed a tumor regression in the BioBran group.

バイオブランのin vivoにおける抗腫瘍効果
Anticancer effect of BioBran :
Ghoneum M., et al, “In vivo Tumor Inhibitory Effects of Nutritional Rice Bran Supplement MGN-3/Biobran on Ehrlich Carcinoma-Bearing Mice”, Nutrition and Cancer, 2008.
▶Influence on liver damage
Male Wistar rats (5 rats/group) were given intraperitoneal administration of 800 mg/kg D-galactosamine to induce liver disorder. Serum GOT and GPT were determined 24 hours after the administration of D-galactosamine, as induces of liver damage. One hour before administration of D-galactosamine, 20, 40, or 80 mg/kg of BioBran was administered intraperitoneally. The serum GOT and GPT in the control group without BioBran at 24 hours after the administration of D-galactosamine were 1410 IU/L and 445 IU/L, respectively. Compared to these values, serum GOT and GPT in the groups treated with BioBran were significantly lower at all doses.

Influence on liver damage :
Sanada H. and Y. Egashira, 7th meeting of the Japanese Association for Dietary Fiber Research, Tokyo, 2002.
▶BioBran in combination with a chemotherapeutic agent
Human breast cancer cells (MCF-7) (1 x 104 cells) were seeded into the wells of a plate and incubated for 3 days in the presence of differing concentrations of BioBran, and the chemotherapeutic agent daunorubicin (DNR). Viable cells were counted by the MTT method, and the concentration of DNR which reduced the number of viable cancer cells by 50% (IC50) was determined. DNR inhibited the survival of MCF-7 cells in a concentration-dependent manner, with the IC50 being 1μM. When MCF-7 cells were cultured in the presence of BioBran and DNR, the IC50 of DNR for MCF-7 was reduced significantly (IC50: 0.2μM).

BioBran in combination with a chemotherapeutic agent :
Ghoneum M. and S. Gollapudi, “MGN-3/Biobran, modified arabinoxylan from rice bran, sensitizes human breast cancer cells to chemotherapeutic agent, daunorubicin”, Cancer Detection and Prevention, 2008.
▶Effect of BioBran on survival and quality of life improvement in patients with progressive cancer
Two hundred and five patients with malignant tumors who were receiving treatment with alternative therapy and chemotherapy with mild adverse drug reactions were allocated into two groups. One of the groups (the control group) received conventional alternative therapy and chemotherapy, and the other group (BioBran group) was given 1 g of BioBran three times a day after each meal for 18 months, in addition to receiving the same therapy as the control group. The correlation between natural killer (NK) cell activity determined during the observation period and survival rate was studied.
The patients’ quality of life was assessed on a scale of 0 to 4 with regard to “pain”, “fatigue”, and “nausea” and 0 to 3 with regard to “appetite” at the start of, and during, the observation period. Of the 205 patients participating in the study, 53 patients of the control group could not continue the conventional alternative therapy and dropped out, and therefore 152 patients (56 patients in the control group and 96 in the BioBran group) were included in the analysis. The survival rates at the end of the observation period in the control group and the BioBran group were 35.8% and 54.2%, respectively. This represents a 50% higher survival rate in the BioBran group compared to the control group. It was also shown that higher NK cell activity was associated with a higher survival rate (Table 1). It was confirmed that the patients’ quality of life after the study had improved compared to the quality of life at the start of the study in both the control group and BioBran group; a notable improvement in appetite was observed in the BioBran group (Table 2).

Effect of BioBran on survival and quality of life improvement in patients with progressive cancer :
Takahara K., et al, “The Life Prolongation and QOL Improvement Effect of Rice Bran Arabinoxylan Derivative (MGN-3, BioBran) for Progressive Cancer”, Clinical Pharmacology and Therapy, 2004

No comments:
Post a Comment