What you need to know about Sinovac vaccine
Since 2 June 2021, the Sinovac-Coronavac (Sinovac) Covid-19 vaccine can be administered under the Special Access Route (SAR) scheme1. This is part of the overall efforts to enhance vaccination coverage against Covid-19 in Singapore. Sinovac was approved by the World Health Organisation to be on its emergency-use listing on 1 June 2021.
On this page:
What’s different about Sinovac vaccine?
![]()
It is an inactivated virus
Inactivated viruses has been used as vaccinations for more than a century. Some common vaccinations that are inactivated viruses include HPV vaccine, flu vaccines and hepatitis A.
![]()
It uses your own antibodies against COVID-19
Sinovac works by teaching your immune system to make antibodies against Covid-19.
![]()
It is stored at normal fridge temperatures
Like the traditional flu vaccination, Sinovac is stored at normal fridge temperatures of 2 to 8°C.
Who is eligible for the Sinovac vaccine?
| Eligible for use in adults 18 years and older. | |
| Suitable for consideration by individuals who are not able to take mRNA vaccines due to anaphylaxis. Based on existing clinical trials and studies, no severe hypersensitivity and anaphylaxis reactions were recorded. |
How does Sinovac work to protect me against COVID-19?
The Sinovac vaccine is a vaccine that uses inactivated COVID-19 virus to teach your immune system to make antibodies against COVID-19, creating an immune response to COVID-19.
When introduced to your body, your immune system will make antibodies against the coronavirus, attaching to viral proteins. As the vaccine uses a dead or inactivated virus, the vaccine can be safely injected into the arm without causing the individual to develop COVID-19.
Once the vaccine is in your system, some of the inactivated viruses will be absorbed by a type of immune cell called the anti-gen presenting cell. This type of cell will then display some of the fragments of inactivated virus on its surface, then known as a helper T cell. If the fragment fits onto the surface protein of the cell, it then can be activated and gets other immune cells to also respond to the vaccine.
When you are vaccinated with Sinovac, your body’s immune system can respond to an infection of live coronaviruses by producing antibodies to block the virus.
How safe is Sinovac?
The WHO recommends the use of the Sinovac vaccine for adults 18 years and older via a two-dose schedule with a window of two to four weeks between doses. According to WHO vaccine efficacy studies showed that the vaccine has a 51% efficacy in preventing Covid-19 and has prevented severe Covid-19 and hospitalisation in 100% of the studied population2.
Approval of Sinovac Covid-19 Vaccine in Countries:
China’s Sinovac-Biotech Covid-19 vaccine has been approved by the World Health Organisation for emergency use in adults 18 years or older. There were no safety concerns identified from pre-clinical trials, while most adverse events were mild to moderate - pain at the injection spot, headache, fatigue. Although efficacy rates differ in countries that participated in the clinical trials, the vaccine efficacy results were promising, showing that the Sinovac vaccine prevented severe COVID-19 symptoms in 100 percent of the studied population.
 | |
| Participating Countries In Clinical Efficacy Trials | Efficacy Rate |
| Brazil | 50.65% |
| Chile | 67% |
| Indonesia | 65.3% |
| Turkey | 83.5% |
| Countries That Approve Emergency Use of Sinova | |
| Albania, Azerbaijan, Brazil, Cambodia, Chile, China, Colombia, Dominican Republic, Ecuador, Egypt, El Salvador, Hong Kong, Indonesia, Lao People's Democratic Republic, Malaysia, Mexico, Pakistan, Panama, Philippines, Thailand, Tunisia, Turkey, Ukraine, Uruguay, Zimbabwe | |
(Source: Channel News Asia, World Health Organisation, US Centers For Disease Control and Prevention)

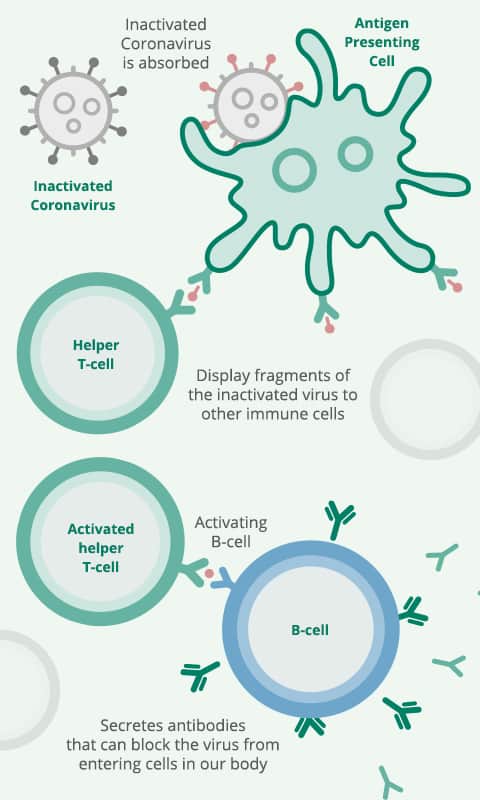 Once the vaccine is in your system, some of the inactivated viruses will be absorbed by a type of immune cell called the anti-gen presenting cell. This type of cell will then display some of the fragments of inactivated virus on its surface, then known as a helper T cell. If the fragment fits onto the surface protein of the cell, it then can be activated and gets other immune cells to also respond to the vaccine.
Once the vaccine is in your system, some of the inactivated viruses will be absorbed by a type of immune cell called the anti-gen presenting cell. This type of cell will then display some of the fragments of inactivated virus on its surface, then known as a helper T cell. If the fragment fits onto the surface protein of the cell, it then can be activated and gets other immune cells to also respond to the vaccine.






No comments:
Post a Comment