Within the pancreas there are areas that are called the islets of Langerhans. The beta cells constitute the predominant type of cell in the islets. The beta cells are particularly important because they make insulin. Degeneration of the beta cells is the main cause of type I (insulin-dependent) diabetes mellitus.
How do beta cells produce insulin?
The primary function of a beta cell is to store and release insulin. Insulin is a hormone that brings about effects which reduce blood glucose concentration. Beta cells can respond quickly to spikes in blood glucose concentrations by secreting some of their stored insulin while simultaneously producing more.
What is an islet cell?
Pancreatic islets, also called islets of Langerhans, are tiny clusters of cells scattered throughout the pancreas. Pancreatic islets contain several types of cells, including beta cells, that produce the hormone insulin. Insulin helps cells throughout the body absorb glucose from the bloodstream and use it for energy.
Which cells do not need insulin?
It should be noted here that there are some tissues that do not require insulin for efficient uptake of glucose: important examples are brain and the liver. This is because these cells don't use GLUT4 for importing glucose, but rather, another transporter that is not insulin-dependent.
What is beta cell strain?
Beta cells produce insulin, and also secrete insulin when they are signaled to do so by an increase in glucose levels in the blood. Without adequate insulin, blood glucose levels rise too high, a defining characteristic of any type of diabetes.
Where are the islets of Langerhans located and what is their function?

The pancreatic islets or islets of Langerhans are the regions of the pancreas that contain its endocrine (i.e., hormone-producing) cells, discovered in 1869 by German pathological anatomist Paul Langerhans.






How Insulin is synthesized?
Insulin is synthesized in significant quantities only in beta cells in the pancreas. ... When the beta cell is appropriately stimulated, insulin is secreted from the cell by exocytosis and diffuses into islet capillary blood. C peptide is also secreted into blood, but has no known biological activity.
Can the beta cells regenerate?
A chemical found in ayahuasca has the potential to regenerate pancreas cells that have been lost to diabetes. New research published in Nature Medicine may have unlocked a new line of treatment for diabetes. The researchers honed in on the main culprits in diabetes: beta cells. Ayahuasca is an Amazonian plant mixture that is capable of inducing altered states of consciousness, usually lasting between 4 to 8 hours after ingestion.
What are the alpha cells?
Alpha cells (more commonly alpha-cells or α-cells) are endocrine cells in the pancreatic islets of the pancreas. They make up to 20% of the human islet cells synthesizing and secreting the peptide hormone glucagon, which elevates the glucose levels in the blood.
Physiologic Effects of Insulin
Stand on a streetcorner and ask people if they know what insulin is, and many will reply, "Doesn't it have something to do with blood sugar?" Indeed, that is correct, but such a response is a bit like saying "Mozart? Wasn't he some kind of a musician?"
Insulin is a key player in the control of intermediary metabolism, and the big picture is that it organizes the use of fuels for either storage or oxidation. Through these activities, insulin has profound effects on both carbohydrate and lipid metabolism, and significant influences on protein and mineral metabolism. Consequently, derangements in insulin signalling have widespread and devastating effects on many organs and tissues.
The Insulin Receptor and Mechanism of Action
Like the receptors for other protein hormones, the receptor for insulin is embedded in the plasma membrane. The insulin receptor is composed of two alpha subunits and two beta subunits linked by disulfide bonds. The alpha chains are entirely extracellular and house insulin binding domains, while the linked beta chains penetrate through the plasma membrane.
The insulin receptor is a tyrosine kinase. In other words, it functions as an enzyme that transfers phosphate groups from ATP to tyrosine residues on intracellular target proteins. Binding of insulin to the alpha subunits causes the beta subunits to phosphorylate themselves (autophosphorylation), thus activating the catalytic activity of the receptor. The activated receptor then phosphorylates a number of intracellular proteins, which in turn alters their activity, thereby generating a biological response.
Several intracellular proteins have been identified as phosphorylation substrates for the insulin receptor, the best-studied of which is insulin receptor substrate 1 or IRS-1. When IRS-1 is activated by phosphorylation, a lot of things happen. Among other things, IRS-1 serves as a type of docking center for recruitment and activation of other enzymes that ultimately mediate insulin's effects. A more detailed look at these processes is presented in the section on Insulin Signal Transduction.
Insulin and Carbohydrate Metabolism
Glucose is liberated from dietary carbohydrate such as starch or sucrose by hydrolysis within the small intestine, and is then absorbed into the blood. Elevated concentrations of glucose in blood stimulate release of insulin, and insulin acts on cells thoughout the body to stimulate uptake, utilization and storage of glucose. The effects of insulin on glucose metabolism vary depending on the target tissue. Two important effects are:
1. Insulin facilitates entry of glucose into muscle, adipose and several other tissues. The only mechanism by which cells can take up glucose is by facilitated diffusion through a family of hexose transporters. In many tissues - muscle being a prime example - the major transporter used for uptake of glucose (called GLUT4) is made available in the plasma membrane through the action of insulin.
When insulin concentrations are low, GLUT4 glucose transporters are present in cytoplasmic vesicles, where they are useless for transporting glucose. Binding of insulin to receptors on such cells leads rapidly to fusion of those vesicles with the plasma membrane and insertion of the glucose transporters, thereby giving the cell an ability to efficiently take up glucose. When blood levels of insulin decrease and insulin receptors are no longer occupied, the glucose transporters are recycled back into the cytoplasm.
It should be noted here that there are some tissues that do not require insulin for efficient uptake of glucose: important examples are brain and the liver. This is because these cells don't use GLUT4 for importing glucose, but rather, another transporter that is not insulin-dependent.
2. Insulin stimulates the liver to store glucose in the form of glycogen. A large fraction of glucose absorbed from the small intestine is immediately taken up by hepatocytes, which convert it into the storage polymer glycogen.
Insulin has several effects in liver which stimulate glycogen synthesis. First, it activates the enzyme hexokinase, which phosphorylates glucose, trapping it within the cell. Coincidently, insulin acts to inhibit the activity of glucose-6-phosphatase. Insulin also activates several of the enzymes that are directly involved in glycogen synthesis, including phosphofructokinase and glycogen synthase. The net effect is clear: when the supply of glucose is abundant, insulin "tells" the liver to bank as much of it as possible for use later.
3. A well-known effect of insulin is to decrease the concentration of glucose in blood, which should make sense considering the mechanisms described above. Another important consideration is that, as blood glucose concentrations fall, insulin secretion ceases. In the absense of insulin, a bulk of the cells in the body become unable to take up glucose, and begin a switch to using alternative fuels like fatty acids for energy. Neurons, however, require a constant supply of glucose, which in the short term, is provided from glycogen reserves.
When insulin levels in blood fall, glycogen synthesis in the liver diminishes and enzymes responsible for breakdown of glycogen become active. Glycogen breakdown is stimulated not only by the absense of insulin but by the presence of glucagon, which is secreted when blood glucose levels fall below the normal range.
Insulin and Lipid Metabolism
The metabolic pathways for utilization of fats and carbohydrates are deeply and intricately intertwined. Considering insulin's profound effects on carbohydrate metabolism, it stands to reason that insulin also has important effects on lipid metabolism, including the following:
1. Insulin promotes synthesis of fatty acids in the liver. As discussed above, insulin is stimulatory to synthesis of glycogen in the liver. However, as glycogen accumulates to high levels (roughly 5% of liver mass), further synthesis is strongly suppressed.
When the liver is saturated with glycogen, any additional glucose taken up by hepatocytes is shunted into pathways leading to synthesis of fatty acids, which are exported from the liver as lipoproteins. The lipoproteins are ripped apart in the circulation, providing free fatty acids for use in other tissues, including adipocytes, which use them to synthesize triglyceride.
2. Insulin inhibits breakdown of fat in adipose tissue by inhibiting the intracellular lipase that hydrolyzes triglycerides to release fatty acids.
Insulin facilitates entry of glucose into adipocytes, and within those cells, glucose can be used to synthesize glycerol. This glycerol, along with the fatty acids delivered from the liver, are used to synthesize triglyceride within the adipocyte. By these mechanisms, insulin is involved in further accumulation of triglyceride in fat cells.
From a whole body perspective, insulin has a fat-sparing effect. Not only does it drive most cells to preferentially oxidize carbohydrates instead of fatty acids for energy, insulin indirectly stimulates accumulation of fat in adipose tissue.
Other Notable Effects of Insulin
In addition to insulin's effect on entry of glucose into cells, it also stimulates the uptake of amino acids, again contributing to its overall anabolic effect. When insulin levels are low, as in the fasting state, the balance is pushed toward intracellular protein degradation.
Insulin also increases the permiability of many cells to potassium, magnesium and phosphate ions. The effect on potassium is clinically important. Insulin activates sodium-potassium ATPases in many cells, causing a flux of potassium into cells. Under certain circumstances, injection of insulin can kill patients because of its ability to acutely suppress plasma potassium concentrations.
Insulin Deficiency and Excess Diseases
Diabetes mellitus, arguably the most important metabolic disease of man, is an insulin deficiency state. It also is a significant cause of disease in dogs and cats. Two principal forms of this disease are recognized:
Type I or insulin-dependent diabetes mellitus is the result of a frank deficiency of insulin. The onset of this disease typically is in childhood. It is due to destruction pancreatic beta cells, most likely the result of autoimmunity to one or more components of those cells. Many of the acute effects of this disease can be controlled by insulin replacement therapy. Maintaining tight control of blood glucose concentrations by monitoring, treatment with insulin and dietary management will minimize the long-term adverse effects of this disorder on blood vessels, nerves and other organ systems, allowing a healthy life.
Type II or non-insulin-dependent diabetes mellitus begins as a syndrome of insulin resistance. That is, target tissues fail to respond appropriately to insulin. Typically, the onset of this disease is in adulthood. Despite monumental research efforts, the precise nature of the defects leading to type II diabetes have been difficult to ascertain, and the pathogenesis of this condition is plainly multifactorial. Obesity is clearly a major risk factor, but in some cases of extreme obesity in humans and animals, insulin sensitivity is normal. Because there is not, at least initially, an inability to secrete adequate amounts of insulin, insulin injections are not useful for therapy. Rather the disease is controlled through dietary therapy and hypoglycemic agents.
Hyperinsulinemia or excessive insulin secretion is most commonly a consequence of insulin resistance, associated with type 2 diabetes or the metabolic syndrome. More rarely, hyperinsulinemia results from an insulin-secreting tumor (insulinoma) in the pancreas. Hyperinsulinemia due to accidental or deliberate injection of excessive insulin is dangerous and can be acutely life-threatening because blood levels of glucose drop rapidly and the brain becomes starved for energy (insulin shock).
Physiologic Effects of Insulin
Stand on a streetcorner and ask people if they know what insulin is, and many will reply, "Doesn't it have something to do with blood sugar?" Indeed, that is correct, but such a response is a bit like saying "Mozart? Wasn't he some kind of a musician?"
Insulin is a key player in the control of intermediary metabolism, and the big picture is that it organizes the use of fuels for either storage or oxidation. Through these activities, insulin has profound effects on both carbohydrate and lipid metabolism, and significant influences on protein and mineral metabolism. Consequently, derangements in insulin signalling have widespread and devastating effects on many organs and tissues.
The Insulin Receptor and Mechanism of Action
Like the receptors for other protein hormones, the receptor for insulin is embedded in the plasma membrane. The insulin receptor is composed of two alpha subunits and two beta subunits linked by disulfide bonds. The alpha chains are entirely extracellular and house insulin binding domains, while the linked beta chains penetrate through the plasma membrane.
The insulin receptor is a tyrosine kinase. In other words, it functions as an enzyme that transfers phosphate groups from ATP to tyrosine residues on intracellular target proteins. Binding of insulin to the alpha subunits causes the beta subunits to phosphorylate themselves (autophosphorylation), thus activating the catalytic activity of the receptor. The activated receptor then phosphorylates a number of intracellular proteins, which in turn alters their activity, thereby generating a biological response.
Several intracellular proteins have been identified as phosphorylation substrates for the insulin receptor, the best-studied of which is insulin receptor substrate 1 or IRS-1. When IRS-1 is activated by phosphorylation, a lot of things happen. Among other things, IRS-1 serves as a type of docking center for recruitment and activation of other enzymes that ultimately mediate insulin's effects. A more detailed look at these processes is presented in the section on Insulin Signal Transduction.
Insulin and Carbohydrate Metabolism
Glucose is liberated from dietary carbohydrate such as starch or sucrose by hydrolysis within the small intestine, and is then absorbed into the blood. Elevated concentrations of glucose in blood stimulate release of insulin, and insulin acts on cells thoughout the body to stimulate uptake, utilization and storage of glucose. The effects of insulin on glucose metabolism vary depending on the target tissue. Two important effects are:
1. Insulin facilitates entry of glucose into muscle, adipose and several other tissues. The only mechanism by which cells can take up glucose is by facilitated diffusion through a family of hexose transporters. In many tissues - muscle being a prime example - the major transporter used for uptake of glucose (called GLUT4) is made available in the plasma membrane through the action of insulin.
When insulin concentrations are low, GLUT4 glucose transporters are present in cytoplasmic vesicles, where they are useless for transporting glucose. Binding of insulin to receptors on such cells leads rapidly to fusion of those vesicles with the plasma membrane and insertion of the glucose transporters, thereby giving the cell an ability to efficiently take up glucose. When blood levels of insulin decrease and insulin receptors are no longer occupied, the glucose transporters are recycled back into the cytoplasm.
It should be noted here that there are some tissues that do not require insulin for efficient uptake of glucose: important examples are brain and the liver. This is because these cells don't use GLUT4 for importing glucose, but rather, another transporter that is not insulin-dependent.
2. Insulin stimulates the liver to store glucose in the form of glycogen. A large fraction of glucose absorbed from the small intestine is immediately taken up by hepatocytes, which convert it into the storage polymer glycogen.
Insulin has several effects in liver which stimulate glycogen synthesis. First, it activates the enzyme hexokinase, which phosphorylates glucose, trapping it within the cell. Coincidently, insulin acts to inhibit the activity of glucose-6-phosphatase. Insulin also activates several of the enzymes that are directly involved in glycogen synthesis, including phosphofructokinase and glycogen synthase. The net effect is clear: when the supply of glucose is abundant, insulin "tells" the liver to bank as much of it as possible for use later.
3. A well-known effect of insulin is to decrease the concentration of glucose in blood, which should make sense considering the mechanisms described above. Another important consideration is that, as blood glucose concentrations fall, insulin secretion ceases. In the absense of insulin, a bulk of the cells in the body become unable to take up glucose, and begin a switch to using alternative fuels like fatty acids for energy. Neurons, however, require a constant supply of glucose, which in the short term, is provided from glycogen reserves.
When insulin levels in blood fall, glycogen synthesis in the liver diminishes and enzymes responsible for breakdown of glycogen become active. Glycogen breakdown is stimulated not only by the absense of insulin but by the presence of glucagon, which is secreted when blood glucose levels fall below the normal range.
Insulin and Lipid Metabolism
The metabolic pathways for utilization of fats and carbohydrates are deeply and intricately intertwined. Considering insulin's profound effects on carbohydrate metabolism, it stands to reason that insulin also has important effects on lipid metabolism, including the following:
1. Insulin promotes synthesis of fatty acids in the liver. As discussed above, insulin is stimulatory to synthesis of glycogen in the liver. However, as glycogen accumulates to high levels (roughly 5% of liver mass), further synthesis is strongly suppressed.
When the liver is saturated with glycogen, any additional glucose taken up by hepatocytes is shunted into pathways leading to synthesis of fatty acids, which are exported from the liver as lipoproteins. The lipoproteins are ripped apart in the circulation, providing free fatty acids for use in other tissues, including adipocytes, which use them to synthesize triglyceride.
2. Insulin inhibits breakdown of fat in adipose tissue by inhibiting the intracellular lipase that hydrolyzes triglycerides to release fatty acids.
Insulin facilitates entry of glucose into adipocytes, and within those cells, glucose can be used to synthesize glycerol. This glycerol, along with the fatty acids delivered from the liver, are used to synthesize triglyceride within the adipocyte. By these mechanisms, insulin is involved in further accumulation of triglyceride in fat cells.
From a whole body perspective, insulin has a fat-sparing effect. Not only does it drive most cells to preferentially oxidize carbohydrates instead of fatty acids for energy, insulin indirectly stimulates accumulation of fat in adipose tissue.
Other Notable Effects of Insulin
In addition to insulin's effect on entry of glucose into cells, it also stimulates the uptake of amino acids, again contributing to its overall anabolic effect. When insulin levels are low, as in the fasting state, the balance is pushed toward intracellular protein degradation.
Insulin also increases the permiability of many cells to potassium, magnesium and phosphate ions. The effect on potassium is clinically important. Insulin activates sodium-potassium ATPases in many cells, causing a flux of potassium into cells. Under certain circumstances, injection of insulin can kill patients because of its ability to acutely suppress plasma potassium concentrations.
Insulin Deficiency and Excess Diseases
Diabetes mellitus, arguably the most important metabolic disease of man, is an insulin deficiency state. It also is a significant cause of disease in dogs and cats. Two principal forms of this disease are recognized:
Type I or insulin-dependent diabetes mellitus is the result of a frank deficiency of insulin. The onset of this disease typically is in childhood. It is due to destruction pancreatic beta cells, most likely the result of autoimmunity to one or more components of those cells. Many of the acute effects of this disease can be controlled by insulin replacement therapy. Maintaining tight control of blood glucose concentrations by monitoring, treatment with insulin and dietary management will minimize the long-term adverse effects of this disorder on blood vessels, nerves and other organ systems, allowing a healthy life.
Type II or non-insulin-dependent diabetes mellitus begins as a syndrome of insulin resistance. That is, target tissues fail to respond appropriately to insulin. Typically, the onset of this disease is in adulthood. Despite monumental research efforts, the precise nature of the defects leading to type II diabetes have been difficult to ascertain, and the pathogenesis of this condition is plainly multifactorial. Obesity is clearly a major risk factor, but in some cases of extreme obesity in humans and animals, insulin sensitivity is normal. Because there is not, at least initially, an inability to secrete adequate amounts of insulin, insulin injections are not useful for therapy. Rather the disease is controlled through dietary therapy and hypoglycemic agents.
Hyperinsulinemia or excessive insulin secretion is most commonly a consequence of insulin resistance, associated with type 2 diabetes or the metabolic syndrome. More rarely, hyperinsulinemia results from an insulin-secreting tumor (insulinoma) in the pancreas. Hyperinsulinemia due to accidental or deliberate injection of excessive insulin is dangerous and can be acutely life-threatening because blood levels of glucose drop rapidly and the brain becomes starved for energy (insulin shock).
CURE
For those who have been diagnosed with type 1 diabetes, JDRF is funding research towards curing the disease by replacing or renewing insulin-producing cells, and also stopping the body from attacking these cells.
THE BASIC CHALLENGES OF CURING TYPE 1 DIABETES
Type 1 diabetes occurs when the body’s immune system mistakenly attacks itself and destroys beta cells in the pancreas. Beta cells normally produce insulin, a hormone that helps the body turn sugar from food sources into energy for cells throughout the body. But when the immune attack destroys the beta cells, insulin is no longer produced and the sugar stays in the blood where it can cause serious damage to body organs. Because of this, people with type 1 diabetes have to regularly inject insulin in order to stay alive.
To cure someone with type 1 diabetes, two aspects of the disease need to be corrected.
We need to stop the mistaken immune system attack on the insulin-producing beta cells, as well as protecting new beta cells from this ongoing attack (encapsulation).
We need to restore the body’s ability to produce its own insulin, either by making new beta cells from other remaining healthy cells in the pancreas (regeneration) or by making them in a lab or obtaining them from other animals and putting them into the body (replacement).
We have made good advances in identifying new ways to regenerate beta cells, and encapsulating beta cells in a barrier that protects them from further immune attack. Our cure research priorities in FY14 focus on:
Generating new beta cells from alternative cell sources that can be shielded from the immune system
Blocking the autoimmune response
Obtaining new markers to detect the disease at early stages
How do beta cells produce insulin?
The primary function of a beta cell is to store and release insulin. Insulin is a hormone that brings about effects which reduce blood glucose concentration. Beta cells can respond quickly to spikes in blood glucose concentrations by secreting some of their stored insulin while simultaneously producing more.
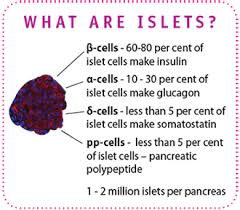
What is an islet cell?
Pancreatic islets, also called islets of Langerhans, are tiny clusters of cells scattered throughout the pancreas. Pancreatic islets contain several types of cells, including beta cells, that produce the hormone insulin. Insulin helps cells throughout the body absorb glucose from the bloodstream and use it for energy.
Which cells do not need insulin?
It should be noted here that there are some tissues that do not require insulin for efficient uptake of glucose: important examples are brain and the liver. This is because these cells don't use GLUT4 for importing glucose, but rather, another transporter that is not insulin-dependent.
What is beta cell strain?
Beta cells produce insulin, and also secrete insulin when they are signaled to do so by an increase in glucose levels in the blood. Without adequate insulin, blood glucose levels rise too high, a defining characteristic of any type of diabetes.
Where are the islets of Langerhans located and what is their function?
The pancreatic islets or islets of Langerhans are the regions of the pancreas that contain its endocrine (i.e., hormone-producing) cells, discovered in 1869 by German pathological anatomist Paul Langerhans.
How Insulin is synthesized?
Insulin is synthesized in significant quantities only in beta cells in the pancreas. ... When the beta cell is appropriately stimulated, insulin is secreted from the cell by exocytosis and diffuses into islet capillary blood. C peptide is also secreted into blood, but has no known biological activity.
Can the beta cells regenerate?
A chemical found in ayahuasca has the potential to regenerate pancreas cells that have been lost to diabetes. New research published in Nature Medicine may have unlocked a new line of treatment for diabetes. The researchers honed in on the main culprits in diabetes: beta cells. Ayahuasca is an Amazonian plant mixture that is capable of inducing altered states of consciousness, usually lasting between 4 to 8 hours after ingestion.
What are the alpha cells?
Alpha cells (more commonly alpha-cells or α-cells) are endocrine cells in the pancreatic islets of the pancreas. They make up to 20% of the human islet cells synthesizing and secreting the peptide hormone glucagon, which elevates the glucose levels in the blood.
Physiologic Effects of Insulin
Stand on a streetcorner and ask people if they know what insulin is, and many will reply, "Doesn't it have something to do with blood sugar?" Indeed, that is correct, but such a response is a bit like saying "Mozart? Wasn't he some kind of a musician?"
Insulin is a key player in the control of intermediary metabolism, and the big picture is that it organizes the use of fuels for either storage or oxidation. Through these activities, insulin has profound effects on both carbohydrate and lipid metabolism, and significant influences on protein and mineral metabolism. Consequently, derangements in insulin signalling have widespread and devastating effects on many organs and tissues.
The Insulin Receptor and Mechanism of Action
Like the receptors for other protein hormones, the receptor for insulin is embedded in the plasma membrane. The insulin receptor is composed of two alpha subunits and two beta subunits linked by disulfide bonds. The alpha chains are entirely extracellular and house insulin binding domains, while the linked beta chains penetrate through the plasma membrane.
The insulin receptor is a tyrosine kinase. In other words, it functions as an enzyme that transfers phosphate groups from ATP to tyrosine residues on intracellular target proteins. Binding of insulin to the alpha subunits causes the beta subunits to phosphorylate themselves (autophosphorylation), thus activating the catalytic activity of the receptor. The activated receptor then phosphorylates a number of intracellular proteins, which in turn alters their activity, thereby generating a biological response.
Several intracellular proteins have been identified as phosphorylation substrates for the insulin receptor, the best-studied of which is insulin receptor substrate 1 or IRS-1. When IRS-1 is activated by phosphorylation, a lot of things happen. Among other things, IRS-1 serves as a type of docking center for recruitment and activation of other enzymes that ultimately mediate insulin's effects. A more detailed look at these processes is presented in the section on Insulin Signal Transduction.
Insulin and Carbohydrate Metabolism
Glucose is liberated from dietary carbohydrate such as starch or sucrose by hydrolysis within the small intestine, and is then absorbed into the blood. Elevated concentrations of glucose in blood stimulate release of insulin, and insulin acts on cells thoughout the body to stimulate uptake, utilization and storage of glucose. The effects of insulin on glucose metabolism vary depending on the target tissue. Two important effects are:
1. Insulin facilitates entry of glucose into muscle, adipose and several other tissues. The only mechanism by which cells can take up glucose is by facilitated diffusion through a family of hexose transporters. In many tissues - muscle being a prime example - the major transporter used for uptake of glucose (called GLUT4) is made available in the plasma membrane through the action of insulin.
When insulin concentrations are low, GLUT4 glucose transporters are present in cytoplasmic vesicles, where they are useless for transporting glucose. Binding of insulin to receptors on such cells leads rapidly to fusion of those vesicles with the plasma membrane and insertion of the glucose transporters, thereby giving the cell an ability to efficiently take up glucose. When blood levels of insulin decrease and insulin receptors are no longer occupied, the glucose transporters are recycled back into the cytoplasm.
It should be noted here that there are some tissues that do not require insulin for efficient uptake of glucose: important examples are brain and the liver. This is because these cells don't use GLUT4 for importing glucose, but rather, another transporter that is not insulin-dependent.
2. Insulin stimulates the liver to store glucose in the form of glycogen. A large fraction of glucose absorbed from the small intestine is immediately taken up by hepatocytes, which convert it into the storage polymer glycogen.
Insulin has several effects in liver which stimulate glycogen synthesis. First, it activates the enzyme hexokinase, which phosphorylates glucose, trapping it within the cell. Coincidently, insulin acts to inhibit the activity of glucose-6-phosphatase. Insulin also activates several of the enzymes that are directly involved in glycogen synthesis, including phosphofructokinase and glycogen synthase. The net effect is clear: when the supply of glucose is abundant, insulin "tells" the liver to bank as much of it as possible for use later.
3. A well-known effect of insulin is to decrease the concentration of glucose in blood, which should make sense considering the mechanisms described above. Another important consideration is that, as blood glucose concentrations fall, insulin secretion ceases. In the absense of insulin, a bulk of the cells in the body become unable to take up glucose, and begin a switch to using alternative fuels like fatty acids for energy. Neurons, however, require a constant supply of glucose, which in the short term, is provided from glycogen reserves.
When insulin levels in blood fall, glycogen synthesis in the liver diminishes and enzymes responsible for breakdown of glycogen become active. Glycogen breakdown is stimulated not only by the absense of insulin but by the presence of glucagon, which is secreted when blood glucose levels fall below the normal range.
Insulin and Lipid Metabolism
The metabolic pathways for utilization of fats and carbohydrates are deeply and intricately intertwined. Considering insulin's profound effects on carbohydrate metabolism, it stands to reason that insulin also has important effects on lipid metabolism, including the following:
1. Insulin promotes synthesis of fatty acids in the liver. As discussed above, insulin is stimulatory to synthesis of glycogen in the liver. However, as glycogen accumulates to high levels (roughly 5% of liver mass), further synthesis is strongly suppressed.
When the liver is saturated with glycogen, any additional glucose taken up by hepatocytes is shunted into pathways leading to synthesis of fatty acids, which are exported from the liver as lipoproteins. The lipoproteins are ripped apart in the circulation, providing free fatty acids for use in other tissues, including adipocytes, which use them to synthesize triglyceride.
2. Insulin inhibits breakdown of fat in adipose tissue by inhibiting the intracellular lipase that hydrolyzes triglycerides to release fatty acids.
Insulin facilitates entry of glucose into adipocytes, and within those cells, glucose can be used to synthesize glycerol. This glycerol, along with the fatty acids delivered from the liver, are used to synthesize triglyceride within the adipocyte. By these mechanisms, insulin is involved in further accumulation of triglyceride in fat cells.
From a whole body perspective, insulin has a fat-sparing effect. Not only does it drive most cells to preferentially oxidize carbohydrates instead of fatty acids for energy, insulin indirectly stimulates accumulation of fat in adipose tissue.
Other Notable Effects of Insulin
In addition to insulin's effect on entry of glucose into cells, it also stimulates the uptake of amino acids, again contributing to its overall anabolic effect. When insulin levels are low, as in the fasting state, the balance is pushed toward intracellular protein degradation.
Insulin also increases the permiability of many cells to potassium, magnesium and phosphate ions. The effect on potassium is clinically important. Insulin activates sodium-potassium ATPases in many cells, causing a flux of potassium into cells. Under certain circumstances, injection of insulin can kill patients because of its ability to acutely suppress plasma potassium concentrations.
Insulin Deficiency and Excess Diseases
Diabetes mellitus, arguably the most important metabolic disease of man, is an insulin deficiency state. It also is a significant cause of disease in dogs and cats. Two principal forms of this disease are recognized:
Type I or insulin-dependent diabetes mellitus is the result of a frank deficiency of insulin. The onset of this disease typically is in childhood. It is due to destruction pancreatic beta cells, most likely the result of autoimmunity to one or more components of those cells. Many of the acute effects of this disease can be controlled by insulin replacement therapy. Maintaining tight control of blood glucose concentrations by monitoring, treatment with insulin and dietary management will minimize the long-term adverse effects of this disorder on blood vessels, nerves and other organ systems, allowing a healthy life.
Type II or non-insulin-dependent diabetes mellitus begins as a syndrome of insulin resistance. That is, target tissues fail to respond appropriately to insulin. Typically, the onset of this disease is in adulthood. Despite monumental research efforts, the precise nature of the defects leading to type II diabetes have been difficult to ascertain, and the pathogenesis of this condition is plainly multifactorial. Obesity is clearly a major risk factor, but in some cases of extreme obesity in humans and animals, insulin sensitivity is normal. Because there is not, at least initially, an inability to secrete adequate amounts of insulin, insulin injections are not useful for therapy. Rather the disease is controlled through dietary therapy and hypoglycemic agents.
Hyperinsulinemia or excessive insulin secretion is most commonly a consequence of insulin resistance, associated with type 2 diabetes or the metabolic syndrome. More rarely, hyperinsulinemia results from an insulin-secreting tumor (insulinoma) in the pancreas. Hyperinsulinemia due to accidental or deliberate injection of excessive insulin is dangerous and can be acutely life-threatening because blood levels of glucose drop rapidly and the brain becomes starved for energy (insulin shock).
Physiologic Effects of Insulin
Stand on a streetcorner and ask people if they know what insulin is, and many will reply, "Doesn't it have something to do with blood sugar?" Indeed, that is correct, but such a response is a bit like saying "Mozart? Wasn't he some kind of a musician?"
Insulin is a key player in the control of intermediary metabolism, and the big picture is that it organizes the use of fuels for either storage or oxidation. Through these activities, insulin has profound effects on both carbohydrate and lipid metabolism, and significant influences on protein and mineral metabolism. Consequently, derangements in insulin signalling have widespread and devastating effects on many organs and tissues.
The Insulin Receptor and Mechanism of Action
Like the receptors for other protein hormones, the receptor for insulin is embedded in the plasma membrane. The insulin receptor is composed of two alpha subunits and two beta subunits linked by disulfide bonds. The alpha chains are entirely extracellular and house insulin binding domains, while the linked beta chains penetrate through the plasma membrane.
The insulin receptor is a tyrosine kinase. In other words, it functions as an enzyme that transfers phosphate groups from ATP to tyrosine residues on intracellular target proteins. Binding of insulin to the alpha subunits causes the beta subunits to phosphorylate themselves (autophosphorylation), thus activating the catalytic activity of the receptor. The activated receptor then phosphorylates a number of intracellular proteins, which in turn alters their activity, thereby generating a biological response.
Several intracellular proteins have been identified as phosphorylation substrates for the insulin receptor, the best-studied of which is insulin receptor substrate 1 or IRS-1. When IRS-1 is activated by phosphorylation, a lot of things happen. Among other things, IRS-1 serves as a type of docking center for recruitment and activation of other enzymes that ultimately mediate insulin's effects. A more detailed look at these processes is presented in the section on Insulin Signal Transduction.
Insulin and Carbohydrate Metabolism
Glucose is liberated from dietary carbohydrate such as starch or sucrose by hydrolysis within the small intestine, and is then absorbed into the blood. Elevated concentrations of glucose in blood stimulate release of insulin, and insulin acts on cells thoughout the body to stimulate uptake, utilization and storage of glucose. The effects of insulin on glucose metabolism vary depending on the target tissue. Two important effects are:
1. Insulin facilitates entry of glucose into muscle, adipose and several other tissues. The only mechanism by which cells can take up glucose is by facilitated diffusion through a family of hexose transporters. In many tissues - muscle being a prime example - the major transporter used for uptake of glucose (called GLUT4) is made available in the plasma membrane through the action of insulin.
When insulin concentrations are low, GLUT4 glucose transporters are present in cytoplasmic vesicles, where they are useless for transporting glucose. Binding of insulin to receptors on such cells leads rapidly to fusion of those vesicles with the plasma membrane and insertion of the glucose transporters, thereby giving the cell an ability to efficiently take up glucose. When blood levels of insulin decrease and insulin receptors are no longer occupied, the glucose transporters are recycled back into the cytoplasm.
It should be noted here that there are some tissues that do not require insulin for efficient uptake of glucose: important examples are brain and the liver. This is because these cells don't use GLUT4 for importing glucose, but rather, another transporter that is not insulin-dependent.
2. Insulin stimulates the liver to store glucose in the form of glycogen. A large fraction of glucose absorbed from the small intestine is immediately taken up by hepatocytes, which convert it into the storage polymer glycogen.
Insulin has several effects in liver which stimulate glycogen synthesis. First, it activates the enzyme hexokinase, which phosphorylates glucose, trapping it within the cell. Coincidently, insulin acts to inhibit the activity of glucose-6-phosphatase. Insulin also activates several of the enzymes that are directly involved in glycogen synthesis, including phosphofructokinase and glycogen synthase. The net effect is clear: when the supply of glucose is abundant, insulin "tells" the liver to bank as much of it as possible for use later.
3. A well-known effect of insulin is to decrease the concentration of glucose in blood, which should make sense considering the mechanisms described above. Another important consideration is that, as blood glucose concentrations fall, insulin secretion ceases. In the absense of insulin, a bulk of the cells in the body become unable to take up glucose, and begin a switch to using alternative fuels like fatty acids for energy. Neurons, however, require a constant supply of glucose, which in the short term, is provided from glycogen reserves.
When insulin levels in blood fall, glycogen synthesis in the liver diminishes and enzymes responsible for breakdown of glycogen become active. Glycogen breakdown is stimulated not only by the absense of insulin but by the presence of glucagon, which is secreted when blood glucose levels fall below the normal range.
Insulin and Lipid Metabolism
The metabolic pathways for utilization of fats and carbohydrates are deeply and intricately intertwined. Considering insulin's profound effects on carbohydrate metabolism, it stands to reason that insulin also has important effects on lipid metabolism, including the following:
1. Insulin promotes synthesis of fatty acids in the liver. As discussed above, insulin is stimulatory to synthesis of glycogen in the liver. However, as glycogen accumulates to high levels (roughly 5% of liver mass), further synthesis is strongly suppressed.
When the liver is saturated with glycogen, any additional glucose taken up by hepatocytes is shunted into pathways leading to synthesis of fatty acids, which are exported from the liver as lipoproteins. The lipoproteins are ripped apart in the circulation, providing free fatty acids for use in other tissues, including adipocytes, which use them to synthesize triglyceride.
2. Insulin inhibits breakdown of fat in adipose tissue by inhibiting the intracellular lipase that hydrolyzes triglycerides to release fatty acids.
Insulin facilitates entry of glucose into adipocytes, and within those cells, glucose can be used to synthesize glycerol. This glycerol, along with the fatty acids delivered from the liver, are used to synthesize triglyceride within the adipocyte. By these mechanisms, insulin is involved in further accumulation of triglyceride in fat cells.
From a whole body perspective, insulin has a fat-sparing effect. Not only does it drive most cells to preferentially oxidize carbohydrates instead of fatty acids for energy, insulin indirectly stimulates accumulation of fat in adipose tissue.
Other Notable Effects of Insulin
In addition to insulin's effect on entry of glucose into cells, it also stimulates the uptake of amino acids, again contributing to its overall anabolic effect. When insulin levels are low, as in the fasting state, the balance is pushed toward intracellular protein degradation.
Insulin also increases the permiability of many cells to potassium, magnesium and phosphate ions. The effect on potassium is clinically important. Insulin activates sodium-potassium ATPases in many cells, causing a flux of potassium into cells. Under certain circumstances, injection of insulin can kill patients because of its ability to acutely suppress plasma potassium concentrations.
Insulin Deficiency and Excess Diseases
Diabetes mellitus, arguably the most important metabolic disease of man, is an insulin deficiency state. It also is a significant cause of disease in dogs and cats. Two principal forms of this disease are recognized:
Type I or insulin-dependent diabetes mellitus is the result of a frank deficiency of insulin. The onset of this disease typically is in childhood. It is due to destruction pancreatic beta cells, most likely the result of autoimmunity to one or more components of those cells. Many of the acute effects of this disease can be controlled by insulin replacement therapy. Maintaining tight control of blood glucose concentrations by monitoring, treatment with insulin and dietary management will minimize the long-term adverse effects of this disorder on blood vessels, nerves and other organ systems, allowing a healthy life.
Type II or non-insulin-dependent diabetes mellitus begins as a syndrome of insulin resistance. That is, target tissues fail to respond appropriately to insulin. Typically, the onset of this disease is in adulthood. Despite monumental research efforts, the precise nature of the defects leading to type II diabetes have been difficult to ascertain, and the pathogenesis of this condition is plainly multifactorial. Obesity is clearly a major risk factor, but in some cases of extreme obesity in humans and animals, insulin sensitivity is normal. Because there is not, at least initially, an inability to secrete adequate amounts of insulin, insulin injections are not useful for therapy. Rather the disease is controlled through dietary therapy and hypoglycemic agents.
Hyperinsulinemia or excessive insulin secretion is most commonly a consequence of insulin resistance, associated with type 2 diabetes or the metabolic syndrome. More rarely, hyperinsulinemia results from an insulin-secreting tumor (insulinoma) in the pancreas. Hyperinsulinemia due to accidental or deliberate injection of excessive insulin is dangerous and can be acutely life-threatening because blood levels of glucose drop rapidly and the brain becomes starved for energy (insulin shock).
CURE
For those who have been diagnosed with type 1 diabetes, JDRF is funding research towards curing the disease by replacing or renewing insulin-producing cells, and also stopping the body from attacking these cells.
THE BASIC CHALLENGES OF CURING TYPE 1 DIABETES
Type 1 diabetes occurs when the body’s immune system mistakenly attacks itself and destroys beta cells in the pancreas. Beta cells normally produce insulin, a hormone that helps the body turn sugar from food sources into energy for cells throughout the body. But when the immune attack destroys the beta cells, insulin is no longer produced and the sugar stays in the blood where it can cause serious damage to body organs. Because of this, people with type 1 diabetes have to regularly inject insulin in order to stay alive.
To cure someone with type 1 diabetes, two aspects of the disease need to be corrected.
We need to stop the mistaken immune system attack on the insulin-producing beta cells, as well as protecting new beta cells from this ongoing attack (encapsulation).
We need to restore the body’s ability to produce its own insulin, either by making new beta cells from other remaining healthy cells in the pancreas (regeneration) or by making them in a lab or obtaining them from other animals and putting them into the body (replacement).
We have made good advances in identifying new ways to regenerate beta cells, and encapsulating beta cells in a barrier that protects them from further immune attack. Our cure research priorities in FY14 focus on:
Generating new beta cells from alternative cell sources that can be shielded from the immune system
Blocking the autoimmune response
Obtaining new markers to detect the disease at early stages

No comments:
Post a Comment