Sensory pathways include only those routes which conduct information to the conscious cortex of the brain. However, we will use the term in its more loosely and commonly applied context to include input from all receptors, whether their signals reach the conscious level or not.
GENERAL SOMATIC AFFERENT (GSA) PATHWAYS FROM THE BODY
Pain and Temperature
Pain and temperature information from general somatic receptors is conducted over small-diameter (type A delta and type C) GSA fibers of the spinal nerves into the posterior horn of the spinal cord gray matter (Fig-1). These are monopolar neurons with cell bodies in the posterior root ganglia. After entering the cord, the fibers pass up or down in the dorsolateral tract, located between the tip of the posterior horn and the surface of the spinal cord near the posterior root, before finally synapsing in laminae III and IV.
Fig-1
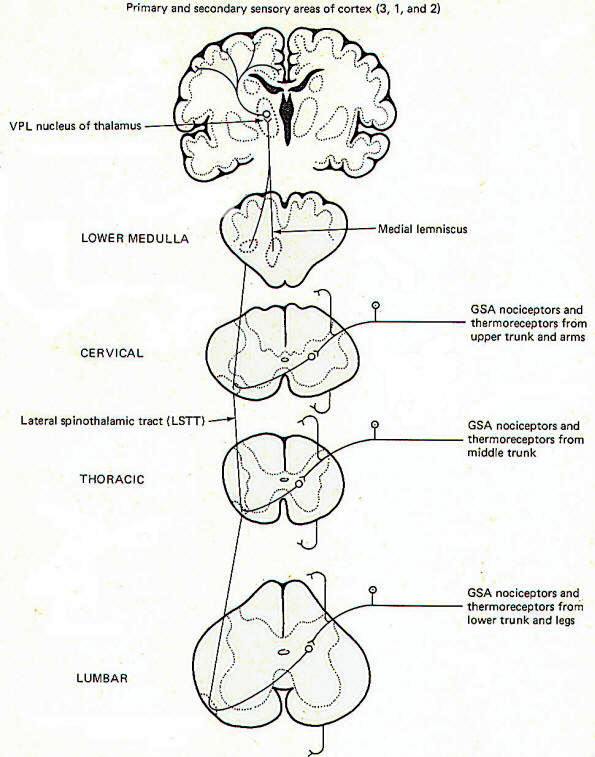
Second-order neurons from these synapses cross over to the opposite side of the cord in the anterior white commissure, where they turn upward as the lateral spinothalamic tract (LSTT). At higher pontine levels this tract comes to lie close to the medial lemniscus, with which it travels to the ventral posterior lateral nucleus (VPL) of the thalamus. Some fibers of this tract don't enter the thalamus but end instead in the brainstem reticular formation. After synapsing in the thalamus, third-order neurons enter the posterior third of the internal capsule, pass through the corona radiata, and terminate in the primary and secondary sensory areas of the parietal lobe cortex (areas 3,1, and 2). Notice that regardless of the level of entry into the spinal cord, pain and temperature stimulation delivered to one side of the body registers in the cerebral cortex of the opposite side.
Fast and Slow Pain
Pain sensation is often confusingly labeled "fast" or "slow" depending on the type of fiber which conducts the impulse and the speed with which the signal consciously registers. Fast pain, often called sharp or pricking pain, is usually conducted to the CNS over type A delta fibers. These ultimately excite lateral spinothalamic tract fibers which go directly to the VPL of the thalamus on the contralateral side. From here third-order fibers project to the cerebral cortex where they are somatotopically organized and sharply localized. Somatotopic organization means that each minute area of the sensory cortex receives input from a distinct peripheral area. A person can sharply localize a pain if he is able to tell exactly where it is originating. Slow pain, often called burning pain, is conducted to the CNS over smaller-diameter type C fibers. After entering the cord these fibers stimulate lateral spinothalamic tract neurons which send collaterals into the brainstem reticular formation. Fibers from the reticular formation diffusely project to the thalamus, hypothalamus, and possibly other areas as well, perhaps giving rise to the emotional component of pain. Pain signals following this route are poorly localized.
Dermatomes
A dermatome is the area of skin supplied by the afferent fibers in the posterior root of a single spinal nerve. Dermatomes tend to overlap each other so that stimulation of a specific point on the skin typically sends afferent signals into the cord over more than one posterior root. This is functionally important since destruction of a single posterior root does not totally eliminate sensation from the afflicted dermatome.
Touch and Pressure
Touch can be subjectively described as discriminating or crude. Discriminating (epicritic) touch implies an awareness of an object's shape, texture, three-dimensional qualities, and other fine points. Also implied here is the ability to recognize familiar objects simply by tactile manipulation. Crude (protopathic) touch, on the other hand, lacks the fine discrimination described above and doesn't generally give enough information to the brain to enable it to recognize a familiar object by touch alone. The tactile information implied here is of a much cruder nature than described for epicritic touch. The pathways to the brain for these two kinds of touch appear to be distinct.
Crude (Protopathic)
Touch and Pressure
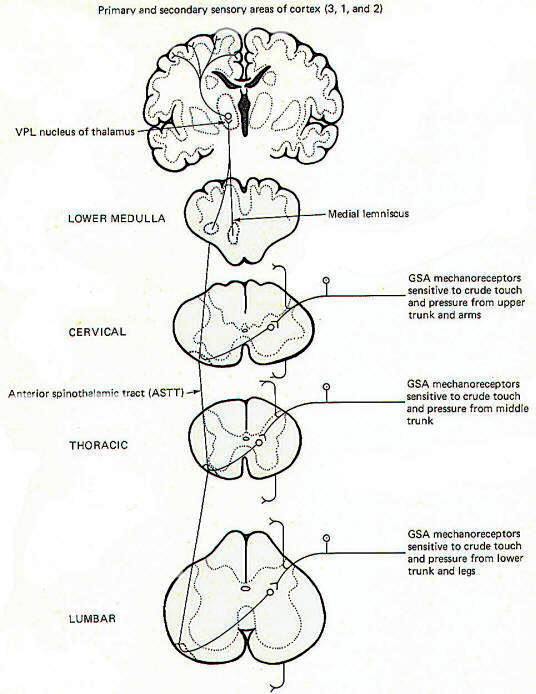
General somatic mechanoreceptors sensitive to crude touch and pressure conduct information into the cord over GSA nerve fibers (Fig-2). The fibers pass up or down a few cord segments (neuromeres) in the dorsolateral (Lissauer) tract before synapsing chiefly in laminae VI, VII, and VIII. Second-order neurons cross over to the opposite side in the anterior white commissure to the anterior funiculus, where they turn upward in the anterior spinothalamic tract (ASTT) to the VPL of the thalamus. At higher pontine levels the tract also comes to lie close to the medial lemniscus as it ascends to the thalamus. Third-order neurons project from the VPL to areas 3, 1, and 2 of the cerebral cortex. Some of the ASTT fibers send collaterals into the brainstem reticular formation. While some of these no doubt ultimately reach the thalamus by reticulothalamic projections, the principal fate and function of these collaterals is largely unknown.
Fig-2
Discriminating (Epicritic) Touch, Pressure, and Kinesthesia
The conscious awareness of body position and movement is called the kinesthetic sense. It's important to recognize that there are many receptors throughout the body which continually conduct information to the brain concerning the body's position and movement and even the level of muscle tone. Such receptors are collectively called proprioceptors. However, not all of these signals reach the conscious level as a large portion are conducted instead to the brainstem and cerebellum for subconscious evaluation and integration. Only those proprioceptive signals reaching the conscious level contribute to the kinesthetic sense. The kinesthetic sense and discriminating touch and pressure pathways share a common route to the brain (Fig-3).
Fig-3

General somatic mechanoreceptors sensitive to discriminating touch and pressure and body position and movement conduct signals into the cord over GSA fibers. They pass directly into the ipsilateral posterior funiculus, where they turn upward in the dorsal columns to terminate in the dorsal column nuclei of the medulla. Those fibers entering the cord below the midthoracic level (i.e., from the lower trunk and legs) ascend through the medial dorsal column as the fasciculus gracilis and terminate in the nucleus gracilis. Fibers entering the cord above the midthoracic level (i.e., from the upper trunk and arms) enter the more lateral dorsal column and ascend as the fasciculus cuneatus to terminate in the more lateral dorsal column nuclei, the nucleus cuneatus. As might be expected, the dorsal columns include the fasciculus gracilis and fasciculus cuneatus while the dorsal column nuclei include the nucleus gracilis and nucleus cuneatus. Second-order neurons from these nuclei cross over to the other side of the brainstem in the lower medulla as the internal arcuate fibers. which then turn upward in the medial lemniscus to the VPL of the thalamus. Third-order neurons then project through the posterior limb of the internal capsule to areas 3, 1, and 2 of the cerebral cortex.
Much of the proprioceptive information which reaches the conscious level giving rise to the kinesthetic sense originates in joint receptors. However, recent evidence indicates that signals from muscle spindles may also represent a significant contribution to kinesthetic sensation. On the other hand, the subconscious proprioceptive information which is shunted to the brainstem and cerebellum for evaluation and integration arises chiefly in muscle spindles and Golgi tendon organs.
Subconscious Proprioception
Most of the subconscious proprioceptive input is shunted to the cerebellum. Further, signals arising in proprioceptors on the left side of the body register on the left side of the cerebellum. By contrast, sensory signals arising in the left side of the body register on the right side of the cerebral cortex. After entering the cord, proprioceptive afferents (GSA fibers) terminate in laminae V, VI, and VII (Clarke's column) of the posterior horn. Second-order neurons (primarily conducting information from Golgi tendon organs) cross over to the opposite side of the cord in the anterior white commissure to the lateral funiculus, where they turn upward in the anterior spinocerebellar tract (ASCT). After reaching upper pontine levels the fibers cross back over and enter the cerebellum through the superior cerebellar peduncle, where they terminate in the vermis (Fig-4). Some of the anterior spinocerebellar tract fibers upon reaching the medulla remain uncrossed and enter the cerebellum via the inferior cerebellar peduncle and terminate in the contralateral vermis. Other second-order neurons (those receiving information primarily from muscle spindles and tendon organs) leave Clarke's column to ascend in the ipsilateral posterior spinocerebellar tract (PSCT) to the cerebellum. After reaching the medulla, the fibers enter the cerebellum via the inferior cerebellar peduncle to terminate in the ipsilateral cortex.
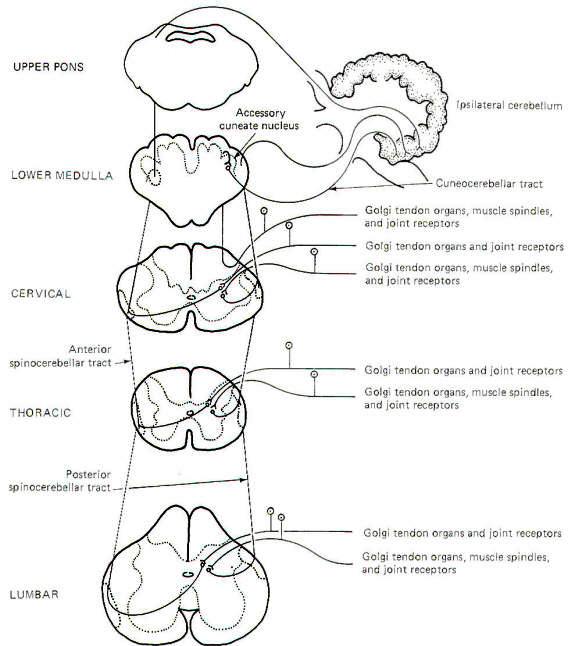
Some of the subconscious proprioceptive input from the cervical region follows an alternate route to the cerebellum. Some of the fibers travel a short distance in the dorsal funiculus, terminating in the accessory cuneate nucleus of the medulla. Second-order neurons project from here as the cuneocerebellar tract to enter the cerebellum via the inferior cerebellar peduncle.
Posterior Funiculus Injury
Certain clinical signs are associated with injury to the dorsal columns. As might be expected, these are generally caused by impairment to the kinesthetic sense and discriminating touch and pressure pathways. They include (1) the inability to recognize limb position, (2) astereognosis, (3) loss of two-point discrimination, (4) loss of vibratory sense, and (5) a positive Romberg sign. Astereognosis is the inability to recognize familiar objects by touch alone. When asked to stand erect with feet together and eyes closed, a person with dorsal column damage may sway and fall. This is a positive Romberg sign.
GENERAL SOMATIC AFFERENT (GSA) PATHWAYS FROM THE FACE
Pain, Temperature, and Crude Touch and Pressure
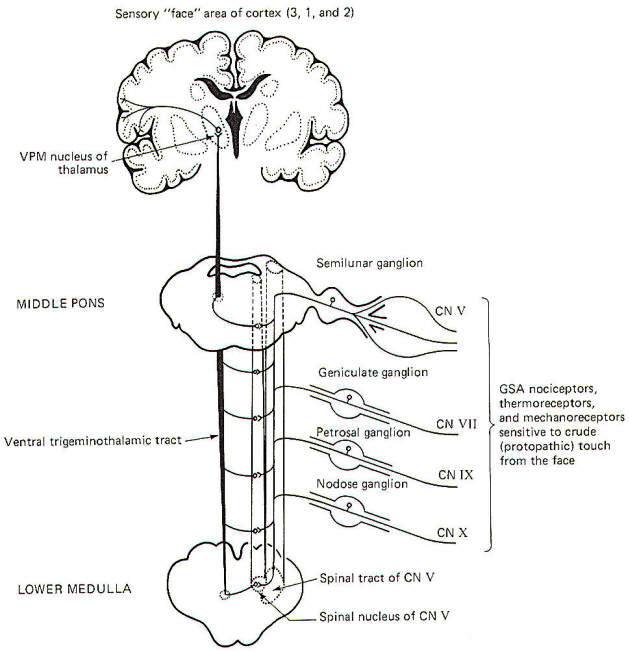
General somatic nociceptors, thermoreceptors, and mechanoreceptors sensitive to crude touch and pressure from the face conduct signals to the brainstem over GSA fibers of cranial nerves V, VII, IX, and X. The afferent fibers involved are processes of monopolar neurons with cell bodies in the semilunar, geniculate, petrosal, and nodose ganglia, respectively. The central processes of these neurons enter the spinal tract of V, where they descend through the brainstem for a short distance before terminating in the spinal nucleus of V. Second-order neurons then cross over the opposite side of the brainstem at various levels to enter the ventral trigeminothalamic tract, where they ascend to the VPM of the thalamus. Finally, third-order neurons project to the "face" area of the cerebral cortex in areas 3, 1, and 2 (Fig-5).
Fig-5
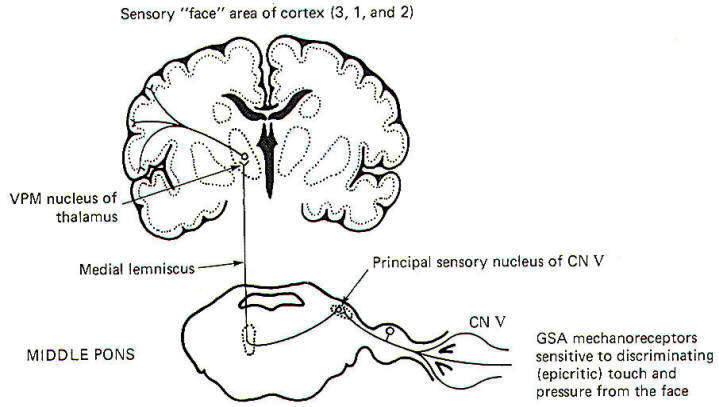
Discriminating Touch and Pressure
The pathway for discriminating touch from the face is illustrated in Fig-6. Signals are conducted from general somatic mechanoreceptors over GSA fibers of the trigeminal nerve into the principal sensory nucleus of V, located in the middle pons. Second-order neurons then conduct the signals to the opposite side of the brainstem, where they ascend in the medial lemniscus to the VPM of the thalamus. Thalamic neurons then project to the "face" region of areas 3, I, and 2 of the cerebral cortex.
Fig-6
Kinesthesia and Subconscious Proprioception
Proprioceptive input from the face is primarily conducted over GSA fibers of the trigeminal nerve. Curiously, however, the cell bodies of these monopolar neurons are located in the mesencephalic nucleus of V in the midbrain rather than the semilunar ganglia, where the cell bodies of other afferent neurons of the trigeminal nerve are located. The peripheral endings of these neurons are the general somatic mechanoreceptors sensitive to both conscious (kinesthetic) and subconscious proprioceptive input. Their central processes extend from the mesencephalic nucleus to the principal sensory nucleus of V in the pons (Fig-7).
Fig-7
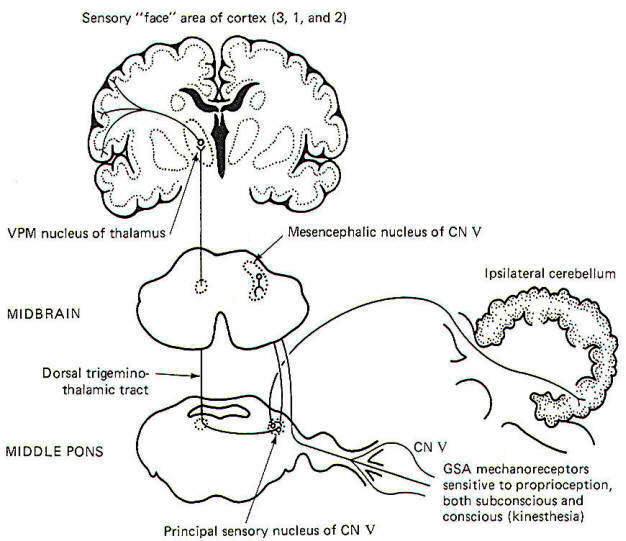
The subconscious component is conducted to the cerebellum, while the conscious component travels to the cerebral cortex. Certain second-order neurons from the principal sensory nucleus relay proprioceptive information concerning subconscious evaluation and integration into the ipsilateral cerebellum. Other second-order neurons project to the opposite side of the pons and ascend to the VPM of the thalamus as the dorsal trigeminothalamic tract. Thalamic projections terminate in the face area of the cerebral cortex.
SPECIAL SOMATIC AFFERENT (SSA) PATHWAYS
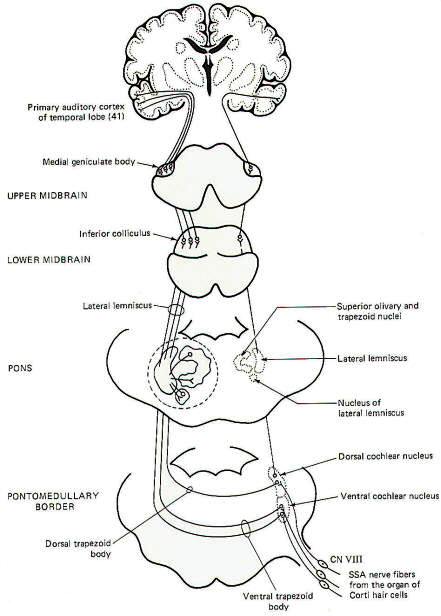
Hearing
The organ of Corti with its sound-sensitive hair cells and basilar membrane are important parts of the sound transducing system for hearing. Mechanical vibrations of the basilar membrane generate membrane potentials in the hair cells which produce impulse patterns in the cochlear portion of the vestibulocochlear nerve (VIII). The principles of this system will be examined elsewhere. For now we will examine only the central pathways from the receptors to their terminations in the brain (Fig-8).
Special somatic nerve fibers of cranial nerve VIII relay impulses from the sound receptors (hair cells) in the cochlear nuclei of the brainstem. These are bipolar neurons with cell bodies located in the spiral ganglia of the cochlea. Their central processes terminate in the dorsal and ventral cochlear nuclei on the ipsilateral side of the brain stem at the pontomedullary border. Most of the second-order neurons arising in the cochlear nuclei cross to the opposite side of the brainstem in the trapezoid body and turn upward in the lateral lemniscus, terminating in the inferior colliculus of the midbrain. Collaterals of the lateral lemniscus terminate in the nucleus of the trapezoid body, superior olivary nucleus, nucleus of the lateral lemniscus, and the brainstem reticular formation. Fibers arising in these nuclei also ascend in the lateral lemniscus. Those fibers from the cochlear nuclei which don't cross over in the trapezoid body ascend in the ipsilateral lateral lemniscus to the inferior colliculus. Sound signals also pass from one side to the other via contralateral projections from one lemniscal nucleus to the other as well as from one inferior colliculus to the other. Thus each lateral lemniscus conducts information from both sides, which helps to explain why damage to a lateral lemniscus produces no appreciable hearing loss other than problems with sound localization. Signals are then conducted from the inferior colliculi to the medial geniculate bodies and finally to the primary auditory area of the temporal lobes (area 41).
Fig-8
Vestibular System
The vestibulocochlear nerve serves two quite different functions. The cochlear portion, previously described, conducts sound information to the brain, while the vestibular portion conducts proprioceptive information. It is the central neural pathways of the latter function which we will examine now (Fig-9). The mechanics and physiology of the vestibular system explained here.
VESTIBULAR SYSTEM
The vestibulocochlear nerve (VIII) has the dual function of serving both the sense of hearing (via cochlear fibers) and proprioception (via vestibular fibers).
THE VESTIBULAR APPARATUS
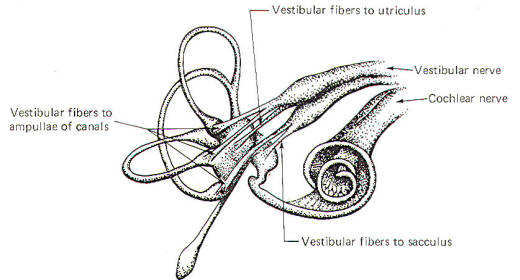
A cavernous network called the bony labyrinth exists within the temporal bone on either side of the head. Within this bony labyrinth is a membranous labyrinth of roughly the same shape filled with endolymph, the same fluid present in the cochlear duct of the inner ear (Fig-1). The endolymph in both the vestibular and cochlear systems is continuous, and is formed in the endolymphatic sac, which makes contact with the fluid of the temporal dura. The space between the membranous and bony labyrinths is filled with perilymph.
The membranous labyrinth is composed of three semicircular canals. Each canal is twice connected to the utriculus, a large endolymph-containing sac. The endolymph of each canal is continuous with that in the utriculus at one end, and separated from it at the other end by a flexible mechanosensitive barrier called the crista ampullaris. The crista is located in the enlarged end of each canal known as the ampulla. The anterior and posterior canals are essentially vertical when a person holds his head erect and they are at right angles to each other. The lateral canal is almost horizontal (actually elevated 23° anteriorly) and forms a plane at right angles to the other two. This geometric arrangement provides the vestibular system with the capability of detecting movements of the head in all directions.
The utriculus is continuous with a second endolymphatic enlargement, the sacculus. A mechanosensitive structure, the macula acustica, is located in the wall of the utriculus with a second macula located in the saccular wall. The three cristae and two maculae are the actual proprioceptive units in each vestibular apparatus. The cristae and maculae are in neural contact with the central nervous system through SSA VIII nerve fibers. Mechanosensitive hair cells in the cristae and maculae form two-element receptors with these fibers.
Fig-1
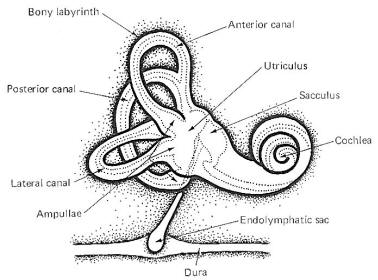
Figure-2 illustrates the distribution of the vestibular nerve fibers to the membranous labyrinth. Notice that one branch of the nerve is distributed to each ampulla, where it distributes to the crista ampullaris hair cells. Separate branches of the nerve are also distributed to the maculae of the utriculus and sacculus, where they form two-element receptors with the macular hair cells.
Fig-2
The Crista Ampullaris
Fig-3
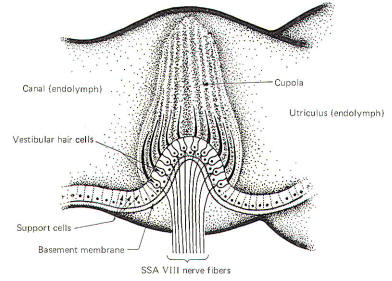
The crista ampullaris is a mechanosensitive flexible barrier to the flow of endolymph between one end of the semicircular canal and the utriculus (Fig-3). A number of sensitive hair cells are interposed with supporting cells at the base of the crista within the ampulla. The hair cell hairs project into a gelatinous mass, the cupola, which projects upward to form a flexible barrier across the space of the ampulla. The cupola behaves like an elastic diaphragm rather than like a swinging door. Angular movements of the head cause the endolymph to push against the cupola so that it bows in one direction or the other. Deflection toward the utriculus is utriculopetal deflection, while deflection away from the utriculus is utriculofugal deflection. Deflecting the cupola bends the hairs, excites the hair cells, and produces impulses in the SSA VIII nerve fibers. In this way the CNS is informed of movements of the head.
There are two types of hair cells in the vestibular apparatus. Type I hair cells are somewhat spherical in shape with 60 to 70 small hairs (stereocilia) emerging from the cuticle (Fig-4). A particularly long hair process, the kinocilium, stands at one end of the stereocilia. Type II hair cells are more cylindrical in shape but their stereocilia and kinocilia are identical with type I cells.
Fig-4
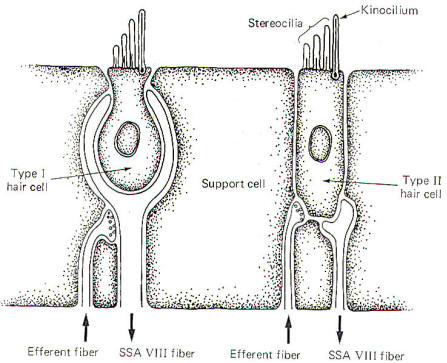
SSA VIII nerve fibers are in close contact with both types of cells, although they form more extensive processes around the base of type I cells. In addition to the SSA fibers, there is evidence that small-diameter efferent fibers of unknown origin also innervate the hair cells. They form direct synaptic contacts with the type II cells but appear instead to terminate on SSA fibers of the type I cells. The origin and function of these efferent fibers is unknown. It seems likely that they may in some way influence the excitability of the hair cells and their potential for producing impulses in the SSA VIII nerve fibers.
Hair Cell Stimulation and Cochlear Nerve Discharge
The stereocilia and kinocilium of each hair cell project up into the gelatinous cupola. Consequently, whenever the cupola is displaced, either toward the utriculus or away from it, the hairs are also deflected. Deflection of the hairs toward the kinocilium produces a change in the hair cell sufficient to increase the firing rate in the SSA VIII nerve fibers. Conversely, deflection away from the kinocilium decreases the firing rate (Fig-5).
Fig-5
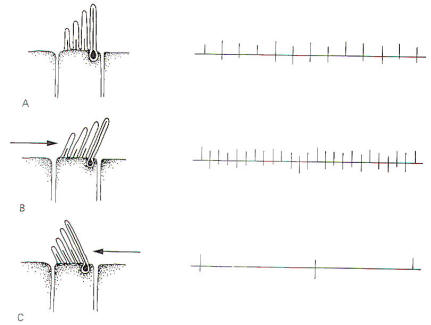
The hair cells in a given crista ampullaris are all orientated in the same direction so that deflection of the cupola either bends all the hairs toward the kinocilia or away from it. Thus deflection of the cupola either increases or decreases the firing rate of the SSA VIII nerve fibers.
In the lateral canals, the kinocilia all face the utriculus. In the vertical canals they all face away from the utriculus, toward the canal. Thus, utriculopetal deflection in the lateral canals produces an increase in the firing rate, while utriculofugal deflection produces a decrease. However, just the opposite is true concerning the vertical canals. Here the hair cell kinocilia are oriented in the opposite direction so that utriculopetal deflection causes a decrease while utriculofugal deflection produces an increase in the firing rate.
Coplanar Canals are Functional Units
The anterior canal on one side of the head and the posterior canal on the opposite side are in the same plane. Thus the two canals are a functional unit since any head movement which causes utriculofugal deflection in the anterior canal on one side will be matched by utriculopetal deflection in the posterior canal on the opposite side (Fig-6). A similar relationship exists with the two lateral canals and they also form a functional unit (Fig-7).
Fig-6
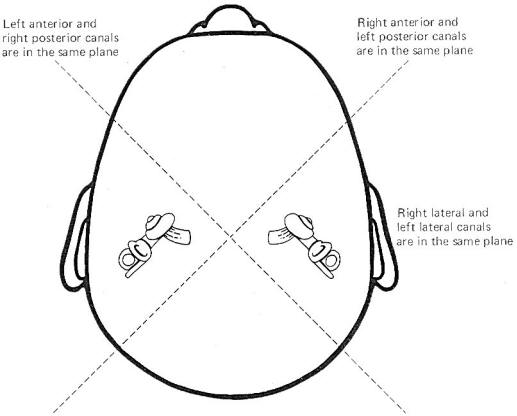
Fig-7
Hair cells stimulate SSA VIII nerve fibers via chemical synapses. Because a fairly steady resting discharge of 40 to 60 impulses per second can be recorded in the nerve fibers, it is assumed that a small amount of transmitter chemical (possibly a catecholamine) is constantly being released. It has been proposed that displacement of the hairs toward the kinocilium increases the firing rate by increasing the rate or amount of transmitter released by the hair cell. Likewise, displacement of the hairs in the opposite direction decreases the firing rate by lowering the rate or amount of release.
In contrast to the stereocilia, which are embedded in the cuticle, the base of the kinocilium is in direct contact with the hair cell cytoplasm. The kinocilium plunging inward (with the aid of the stereocilia leaning against it) may depolarize the hair cell membrane and establish a receptor potential, which in turn causes transmitter release. Alternatively, deflection of the stereocilia away from the kinocilium pulls the kinocilium outward, hyperpolarizing the membrane and decreasing transmitter release.
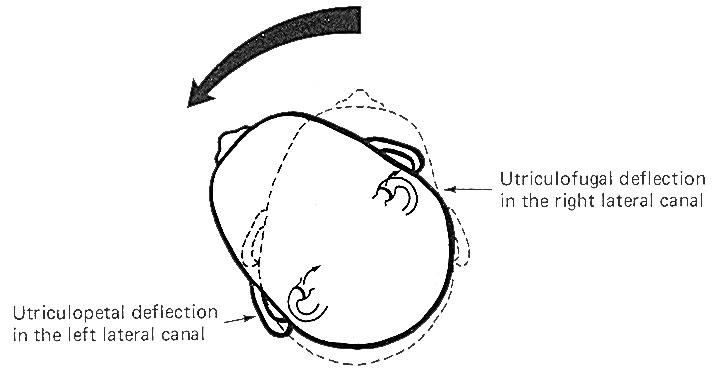
The cristae are particularly sensitive to changes in angular acceleration and deceleration of the head. The greatest change in firing rate along nerve fibers from the cristae occur at the beginning and end of angular movements of the head. As Fig-7 shows, the inertia of the endolymph when the head first starts rotating to the left produces utriculopetal deflection in the left canal and utriculofugal deflection in the right canal. Thus we see a large initial change in firing rate from each canal at the beginning of the movement. However, if the rotation of the head to the left continues, we see no further change in firing rates until the rotation begins to slow down (decelerate). At this point. the inertia of the endolymph causes the cupola to deflect in the opposite direction, once again causing a change in the firing rate. This time, however, there is a decrease in the left canal and an increase in the right canal. Thus one can see that the canal system is particularly adept at signaling changes in acceleration and deceleration of the head's angular movements. Further, because the canals are arranged in three planes, angular movements in all directions are easily detected by the canal system. No doubt angular movements which are not exactly parallel with a single coplanar canal system are detected by the brain through some "weighted" input from two or more coplanar functional units.
The Macula Acustica
The macula acustica is a mechanosensitive structure in the utriculus and sacculus. It is similar to the crista in that the base of the structure is composed of type I and type II hair cells (Fig-8). Likewise, the base of the hair cells forms contacts with SSA VIII nerve fibers. Maculae are also called otolith receptors because the hair processes project into a low-lying gelatinous structure which is impregnated with dense calcareous formations called otoliths or otoconia. The otolith receptors respond to static gravitational pull and are therefore well equipped to signal the position of the head in space at any given time. A basal discharge rate of the SSA VIII fibers from the utriculus is observed when the head is in the normal erect position. This rate increases to a maximum when the head is moved to a position 90° from normal (i.e., 90° forward, backward, or to either side).
In addition to their gravitational or static response, utricular otolith receptors also respond to linear acceleration and deceleration of the head, thus exhibiting a dynamic response characteristic as well. Saccular otolith receptors respond only to the static position of the head in space and demonstrate no appreciable dynamic response.

Fig-8
VESTIBULAR SYSTEM INTERACTIONS
Vestibular Control of Eye Movements
An interesting cooperative relationship exists between the vestibular system and the extraocular muscles of the eye. Those eye movements caused by vestibular stimulation are generally compensatory in nature, attempting to keep to the visual axis relatively fixed when the head is moved in space. This aids both vision and the maintenance of posture. As an example, a cooperative relationship exists between the lateral canals on both sides of the head that is designed to keep the eyes directed toward a reference point in the visual field as the head is moved in a lateral plane (Fig-9). Unless consciously overridden, the eyes move slowly to the left as the head is turned slowly to the right, maintaining a constant reference point in the visual field.
These reflex conjugate eye movements are produced by changes in activity of the extraocular eye muscles in response to vestibular activity. A close examination of Fig-9 shows that when the head is turned to the right, the endolymph in the right lateral canal deflects the cupola toward the utriculus (utriculopetal), while the endolymph of the left lateral canal deflects its cupola away from the utriculus (utriculofugal). Now if we remember that utriculopetal deflection in the lateral canals increases the firing rate while utriculofugal deflection decreases it, an examination of the neural circuitry in Fig-9 explains the slow shift of the eyes to the left. The lateral rectus muscle of the left eye and medial rectus of the right eye both contract, while their antagonists relax, pulling the eyes slowly to the left. A similar cooperative relationship exists between the anterior canal on one side and the posterior canal on the other. The anterior canals are able to produce stimulation of the ipsilateral superior rectus muscle and the contralateral inferior oblique. The posterior canals produce stimulation of the ipsilateral superior oblique and the contralateral inferior rectus muscle. In this way the eyes can maintain their reference point when the head is moved through any plane.
The Vestibulospinal System
While the vestibular system responds primarily to movements of the head, it is able to produce far-reaching postural changes throughout the body. The vestibular system can regulate alpha and gamma motor neuron activity in the spinal cord through the lateral and medial vestibulospinal tracts (Fig-7). The vestibulospinal tracts originate in the vestibular nuclei of the brainstem. Those fibers which originate in the lateral vestibular (Deiter's) nucleus descend ipsilaterally in the anterior funiculus and form the lateral vestibulospinal tract. The fibers of this tract terminate in laminae VII, VIII, and IX at all levels of the cord. Arising from the medial vestibular nucleus are the fibers of the medial vestibulospinal tract. While there is a small crossed component, most of its fibers descend ipsilaterally only as far as the midthoracic level, where they too synapse in laminae VII, VIII, and IX.
The vestibulospinal tracts facilitate extensor and inhibit flexor alpha and gamma motor neurons. Input to the vestibular nuclei via fibers of cranial nerve VIII from the vestibular apparatus presupposes an antigravity or postural role for the vestibulospinal tracts. Activity in these tracts is also influenced by input to the vestibular nuclei from the cerebellum, and through it, the peripheral proprioceptors of muscles, tendons, and joints.
The Vestibular System and the Cerebellum
Because of the role the vestibular system plays in the maintenance of posture and muscle control, it is not surprising to find that the system has a close relationship with the cerebellum. Both first- and second-order vestibulocerebellar fibers end as mossy fibers on the granular cells of the cerebellar cortex of the flocculonodular lobe. In addition, the fastigial and dentate cerebellar nuclei also receive vestibular input. Presumably the cerebellar cortex integrates the vestibular input with other proprioceptive input from all parts of the body. The cerebellum is then in a position to exert influence on the postural musculature via output to the vestibular, reticular, and red nuclei. Vestibulospinal, reticulospinal, and rubrospinal fibers influence muscle activity at the spinal cord level, while cerebellar output through the thalamus to the cerebral cortex modifies motor activity at the cortical source.
Vestibulocortical Projections
In order to be consciously aware of position and movements of the head in space, it is necessary that vestibular information reach the cerebral cortex. The kinesthetic sense (conscious awareness of body position and movement) requires cortical input from peripheral proprioceptors as well as from the vestibular system. The cortical area which receives this information is located in the postcentral gyrus near the somatosensory projection of the mouth. Vestibulocortical projections appear to be primarily contralateral with intermediate synapses in the ipsilateral vestibular nuclei and the contralateral thalamus.
Vestibular System and Autonomic Effects
The effects of vestibular activity on autonomic function are well known and are grouped under the heading "motion sickness." They include effects on the vasomotor system (typically a vasodepressor action with a blood pressure drop), an increase in the rate and depth of respiration, decreased salivation, increased sweating, pupillary dilation, and disturbances of the gastrointestinal tract. Most of these effects are mediated through the sympathetic nervous system.
Tests for the Integrity of the Semicircular Canals
Certain bodily responses to vestibular stimulation are reflexly predictable, such as conjugate movements of the eyes and other postural adjustments of the body. The integrity of the various canals can be tested by their capacity to produce the expected responses. The rotation (swivel chair) test and the caloric test are both designed to do this.
The rotation test allows maximum stimulation of the horizontal and vertical canals. Maximum deflection of the cupola of a particular canal occurs when the movement of the head is in the same plane as the canal which contains that cupola. This is accomplished in the swivel chair by placing the head in various positions and then rotating the chair. Recall that maximum deflection in a canal on one side of the head is accompanied by maximum deflection in its functional counterpart on the opposite side.
Predictable responses observed with rotation tests are nystagmus, vertigo, and past pointing. Nystagmus refers to rapid to-and-fro movements of the eyes. As previously noted, the eyes slowly shift to the left as the head is turned slowly to the right. Of course there is a limit to how far left the eyes can shift if the head continues turning to the right. When they have pulled as far left as possible, they suddenly "snap" back to the right and "fix" on a new reference point in the visual field. This alternating slow phase to the left followed by a fast phase to the right continues as the head keeps rotating to the right unless consciously overridden. While nystagmus technically refers to the eye shifts in both directions, neuroscientists typically refer to nystagmus as the direction of the fast phase. For example, nystagmus is to the right in the case just described.
Because cupola deflection directly controls eye movements, and because this deflection is in one direction during the acceleration phase of the angular rotation and in the opposite direction during the deceleration phase, it follows that nystagmus is in one direction during rotation (perrotation) and in the opposite direction after rotation (postrotation). Perrotational nystagmus is in the same direction as the rotation. However, if the rotating chair is suddenly stopped, the canals cease to rotate but the inertia of the endolymph is not so easily overcome. Consequently the cupolae are deflected in the opposite direction for a brief period of time, producing a postrotational nystagmus in the direction opposite the rotation.
Vertigo and past pointing are also predictably observed following rotation in a normal individual. Vertigo is the sensation of a movement when no such movement exists. This is caused by the fact that once the actual turning stops, the inertia of the endolymph remains for a while, deflecting the cupolae and sending signals to the brain that turning is still occurring. Normally the vertigo (false sense of movement) is in the same direction as the postrotational nystagmus. The body will ordinarily attempt to reflexly make postural adjustments for the vertigo just as it would for a real movement. Thus, predictable leaning of the whole body (a reflex attempt to correct for the false movement) is typically observed following a period of rotation. Specifically, the body leans in the direction opposite the postrotational nystagmus, An extended arm also points in the direction opposite the post rotational nystagmus. This is past pointing,
The rotation test has the disadvantage of not allowing the canals on each side of the head to be tested separately. However, caloric tests, which involve the introduction of hot or cold solutions into the auditory canal, allow the clinician to test each side of the head separately. A hot water solution introduced into the auditory canal causes the endolymph to expand, deflecting the cupola in a predictable direction. This is later followed by the use of a cold water solution which cools the endolymph, producing deflection in the opposite direction. Like the rotation test, predictable changes in nystagmus, vertigo, and past pointing can be observed.
Vestibular System
The vestibulocochlear nerve serves two quite different functions. The cochlear portion, previously described, conducts sound information to the brain, while the vestibular portion conducts proprioceptive information. It is the central neural pathways of the latter function which we will examine now (Fig-9). The mechanics and physiology of the system explained elsewhere.
Fig-9
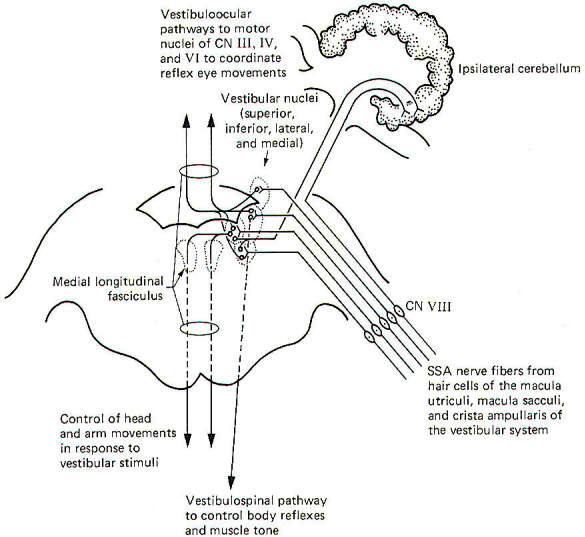
Special somatic afferent fibers from the hair cells of the macula utriculi and macula sacculi conduct information into the vestibular nuclei on the ipsilateral side of the pons and medulla. These are bipolar neurons with cell bodies located in the vestibular ganglion. Some of the fibers project directly into the ipsilateral cerebellum to terminate in the uvula, flocculus, and nodulus, but most enter the vestibular nuclei and synapse there.
As might be expected, neuronal output from the vestibular nuclei effects bodily and eye movements in response to movements of the head as detected by the vestibular apparatus. The vestibulospinal path fibers which affect body reflexes and muscle tone in response to vestibular input originate primarily in the lateral vestibular nucleus. The medial vestibular nucleus is the principal origin of both crossed and uncrossed fibers which descend through the brain stem in the medial longitudinal fasciculus to the upper cord causing various reflex head and arm movements in response to vestibular stimuli. Finally, all four vestibular nuclei (medial, lateral, superior, and inferior) project both crossed and uncrossed fibers to the motor nuclei of cranial nerves Ill, IV, and VI in order to control and coordinate reflex eye movements. These vestibuloocular paths also travel in the medial longitudinal fasciculus.
Vision
The visual system receptors are the rods and cones of the retina. The neurophysiology of vision and visual reflexes are discussed elsewhere. Special somatic afferent fibers of the optic nerve (II) conduct visual signals into the brain. Examination of Fig-10 will show that fibers from the lateral (temporal) retina of either eye terminate in the lateral geniculate body on the same side of the brain as that eye. On the other hand, SSA II fibers from the medial (nasal) retina of each eye cross over in the optic chiasm to terminate in the contralateral lateral geniculate body. The optic nerve is composed of fibers from the retina to the optic chiasm. Even though no synapses occur in the optic chiasm, the continuation of the visual pathway from the optic chiasm to the lateral geniculate body is called the optic tract rather than the optic nerve. After a synapse in the lateral geniculate body, the signal continues in the optic radiation to area 17 of the conscious visual cortex. Area 17 is the primary visual area, which receives initial visual signals. Neurons from this area project into the adjacent occipital cortex (areas 18 and 19) which is known as the secondary visual area. It is here that the visual signal is fully evaluated.
Fig-10
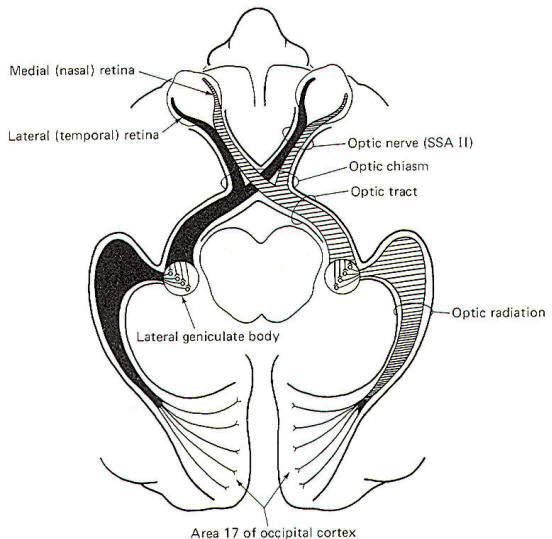
Fig-11
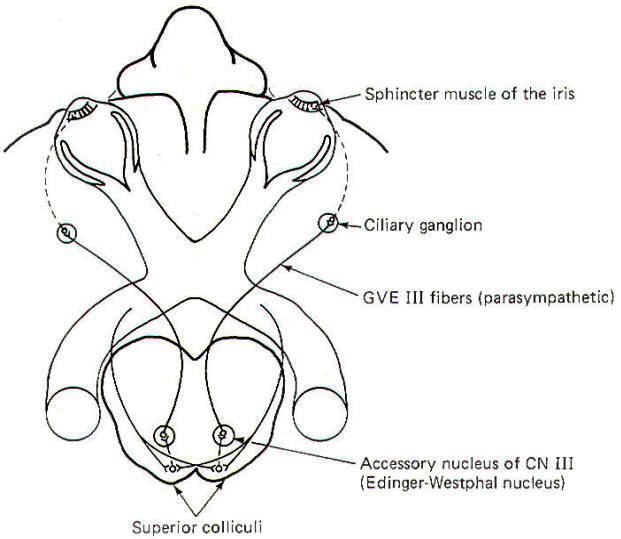
The visual reflex pathway involving the pupillary light reflex is illustrated in Fig-11. This is the well-known reflex in which the pupils constrict when a light is shined into the eyes and dilate when the light is removed. Some SSA II fibers leave the optic tract before reaching the lateral geniculates, terminating in the superior colliculi instead. From here, short neurons project to the EdingerWestphal nucleus (an accessory nucleus of III) in the midbrain, which serves as the origin of the preganglionic parasympathetic fibers of the oculomotor nerve (GVE III). The GVE III fibers in turn project to the ciliary ganglia, from which arise the postganglionic fibers to the sphincter muscles of the iris, which constrict the pupils.
GENERAL VISCERAL AFFERENT (GVA) PATHWAYS
Pain and Pressure Sensation via the Spinal Cord
Visceral pain receptors are located in peritoneal surfaces, pleural membranes, the dura mater, walls of arteries, and the walls of the GI tube. Nociceptors in the walls of the GI tube are particularly sensitive to stretch and overdistension.
General visceral nociceptors conduct signals into the spinal cord over the monopolar neurons of the posterior root ganglia. They terminate in laminae III and IV of the posterior horn as do the pain and temperature pathways of the GSA system; however, their peripheral processes reach the visceral receptors via the gray rami communicantes and ganglia of the sympathetic chain (Fig-12), Second-order neurons from the posterior horn cross in the anterior white commissure and ascend to the thalamus in the anterior and lateral spinothalamic tracts, Projections from the VPL of the thalamus relay signals to the sensory cortex.
Fig-12
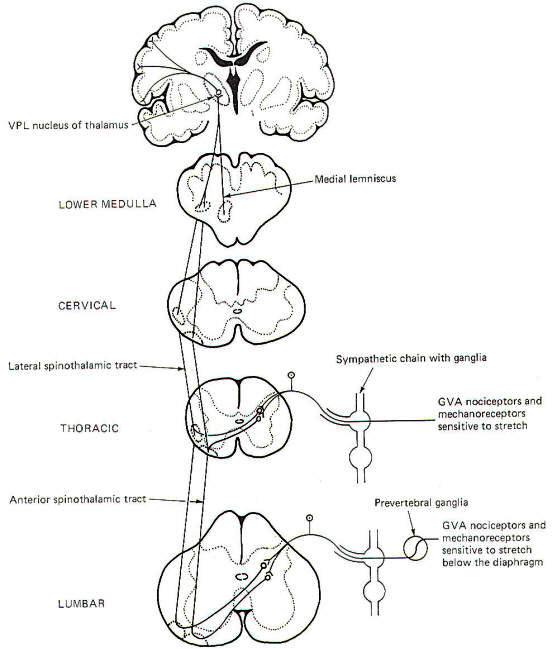
Fig-13
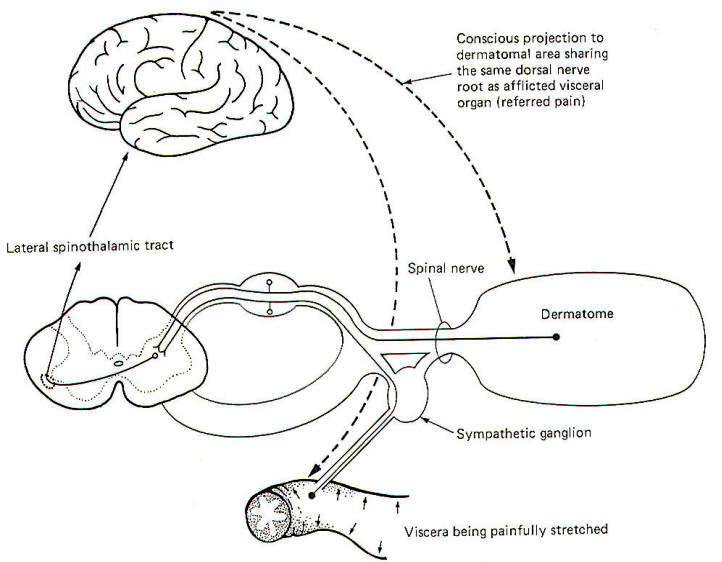
The localization of visceral pain is relatively poor, making it difficult to tell the exact source of the stimuli. At least a partial explanation of our inability to precisely localize visceral pain relates to its rarity. True visceral pain seldom occurs when compared to the frequency of external pain. An additional compounding factor is the phenomena of referred pain. Because true visceral pain is often projected or "referred" by the brain to some area on the surface of the body, its true visceral origin is often confused. The mechanism for referred visceral pain is not fully understood but may result in part from the close proximity in the posterior horn of the central terminals of GVA pain fibers and GSA spinal nerve fibers from the body surface. This is supported by the fact that pain from a visceral origin is referred to a dermatome with which it shares the same posterior root. This is a useful observation, often making it possible to locate the source of a visceral pain from an observation of the surface area to which it is referred. The pain down the inside of the left arm associated with true cardiac pain is a good example.
It is likely that separate second-order neurons relay pain information from GSA and GVA input. If the painful stimulus to the viscera is moderate, the level of activity in the GVA fibers is likely sufficient to stimulate only those second-order neurons which normally relay signals from the viscera. However, if the painful stimulus increases in strength, the increased central synaptic activity of the GVA neurons may "spill over" and raise the central excitatory state of those second-order neurons which normally relay information from GSA fibers of the dermatome. If the painful visceral stimulation is very strong, this "spill over" may be sufficient to exceed the threshold of excitation for these neurons, causing them to fire even though no painful stimulus is delivered to the general somatic nociceptors of the dermatome. Thus the brain incorrectly projects the source of the pain to the dermatomal area (Fig-13).
Blood Pressure, Blood Chemistry, and Alveolar Stretch Detection
The walls of the aorta and the carotid sinuses contain special baroreceptors (pressure receptors) which respond to changes in blood pressure. These mechanoreceptors are the peripheral endings of GVA fibers of the glossopharyngeal (IX) and vagus (X) nerves. The GVA fibers from the carotid sinus baroreceptors enter the solitary tract of the brainstem and terminate in the vasomotor center of the medulla (Fig-14). This is the CNS control center for cardiovascular activity. The cell bodies of these unipolar neurons are located in the petrosal ganglion. GVA fibers of the vagus nerve conduct signals from the baroreceptors in the walls of the aorta to the solitary tract and on to the vasomotor center. The cell bodies of these unipolar neurons are located in the nodose ganglion.
Fig-14
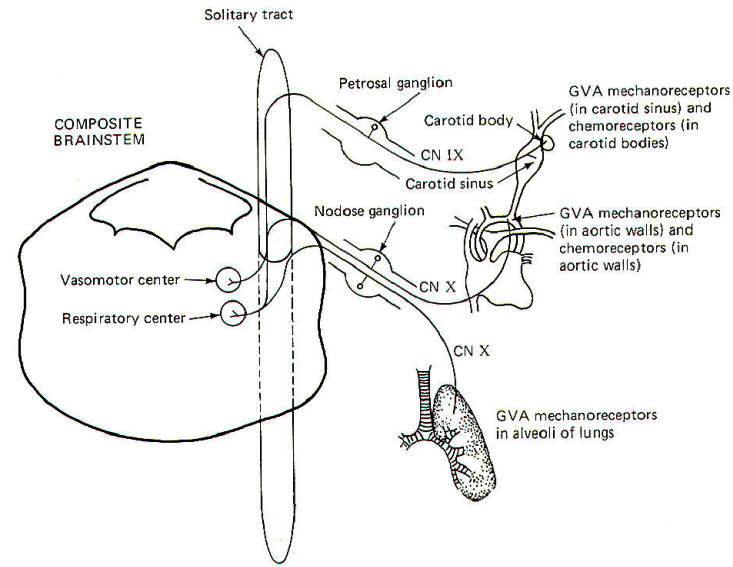
Stretch receptors in the alveoli of the lungs conduct information concerning rhythmic alveolar inflation and deflation over GVA X fibers to the solitary tract and then to the respiratory center of the brainstem. This route is an important link in the Hering-Breuer reflex, which helps to regulate respiration.
Carotid body chemoreceptors, sensitive to changes in blood PO2 and, to a lesser extent, PCO2 and pH, conduct signals to both the vasomotor and respiratory centers over GVA IX nerve fibers. GVA X fibers conduct similar information from the aortic chemoreceptors to both centers. Chemoreceptors were discussed elsewhere.
SPECIAL VISCERAL AFFERENT (SVA) PATHWAYS
Taste
The receptors for taste are the taste cells which produce impulses in afferent fibers in response to chemical stimulation. They were described elsewhere. The pathways for taste sensation are illustrated in Fig-15. Special visceral afferent (SVA) fibers of cranial nerves VII, IX, and X conduct signals into the solitary tract of the brainstem, ultimately terminating in the nucleus of the solitary tract on the ipsilateral side. Second-order neurons cross over and ascend through the brainstem in the medial lemniscus to the VPM of the thalamus. Thalamic projections to area 43 (the primary taste area) of the postcentral gyrus complete the relay. SVA VII fibers conduct from the chemoreceptors of taste buds on the anterior twothirds of the tongue, while SVA IX fibers conduct taste information from buds on the posterior one-third of the tongue. SVA X fibers conduct taste signals from those taste cells located throughout the fauces.
Fig-15
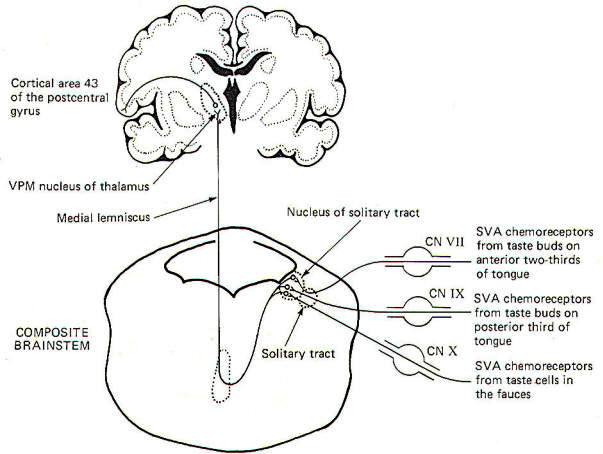
Fig-16
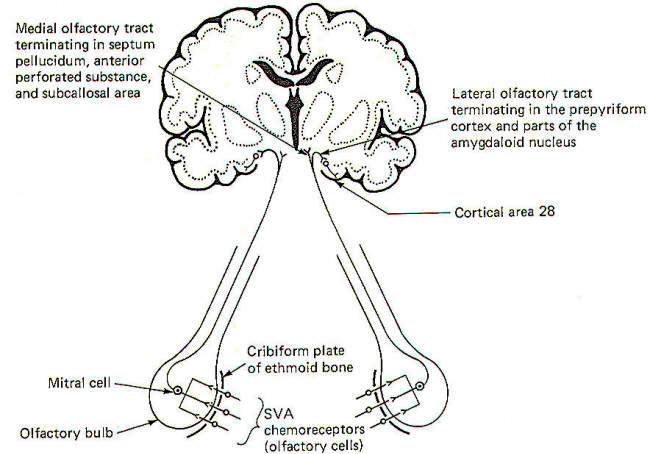
Smell
The sense of smell was examined elsewhere and, once again, we will look only at the central pathways here. The smell-sensitive cells (olfactory cells) of the olfactory epithelium project their central processes through the cribiform plate of the ethmoid bone, where they synapse with mitral cells. The central processes of the mitral cells pass from the olfactory bulb through the olfactory tract, which divides into a medial and lateral portion (Fig-16). The lateral olfactory tract terminates in the prepyriform cortex and parts of the amygdala of the temporal lobe. These areas represent the primary olfactory cortex. Fibers then project from here to area 28, the secondary olfactory area, for sensory evaluation. The medial olfactory tract projects to the anterior perforated substance, the septum pellucidum, the subcallosal area, and even the contralateral olfactory tract. Both the medial and lateral olfactory tracts contribute to the visceral reflex pathways, causing the viscerosomatic and viscerovisceral responses described earlier.
DAMAGE TO THE SPINAL NERVES AND SPINAL CORD
After studying the motor pathways and the sensory pathways, the injuries described in Table-1 would be expected to produce the symptoms listed.
Table-1 Symptoms of Damage to Spinal Nerves and Spinal Cord
Damage Possible cause of damage Symptoms associated with innervated area
1.Peripheral nerve Mechanical injury Loss of muscle tone. Loss of reflexes. Flaccid paralysis. Denervation atrophy. Loss of sensation.
2.Posterior root Tabes dorsalis Paresthesia. Intermittent sharp pains. Decreased sensitivity to pain. Loss of reflexes. Loss of sensation. Positive Romberg sign. High stepping and slapping of feet.
3.Anterior Horn Poliomyelitis Loss of muscle tone. Loss of reflexes. Flaccid paralysis. Denervation atrophy.
4.Lamina X (gray matter) Syringomyelia Bilateral loss of pain and temperature sense only at afflicted cord level. Sensory dissociation. No sensory impairment below afflicted level.
5.Anterior horn and lateral corticospinal tract Amyotrophic lateral sclerosis Muscle weakness. Muscle atrophy. Fasciculations of hand and arm muscles. Spastic paralysis.
6.Posterior and lateral funiculi Subacute combined degeneration. Loss of position sense. Loss of vibratory sense. Positive Romberg sign. Muscle weakness. Spasticity. Hyperactive tendon reflexes. Positive Babinski sign.
7.Hemisection of the spinal cord Mechanical injury Brown-Sequard syndrome.
Below cord level on injured side.
Flaccid paralysis. Hyperactive tendon reflexes. Loss of position sense. Loss of vibratory sense. Tactile impairment.
Below cord level on opposite side beginning one or two segments below injury.
Loss of pain and temperature.
GENERAL SOMATIC AFFERENT (GSA) PATHWAYS FROM THE BODY
Pain and Temperature
Pain and temperature information from general somatic receptors is conducted over small-diameter (type A delta and type C) GSA fibers of the spinal nerves into the posterior horn of the spinal cord gray matter (Fig-1). These are monopolar neurons with cell bodies in the posterior root ganglia. After entering the cord, the fibers pass up or down in the dorsolateral tract, located between the tip of the posterior horn and the surface of the spinal cord near the posterior root, before finally synapsing in laminae III and IV.
Fig-1

Second-order neurons from these synapses cross over to the opposite side of the cord in the anterior white commissure, where they turn upward as the lateral spinothalamic tract (LSTT). At higher pontine levels this tract comes to lie close to the medial lemniscus, with which it travels to the ventral posterior lateral nucleus (VPL) of the thalamus. Some fibers of this tract don't enter the thalamus but end instead in the brainstem reticular formation. After synapsing in the thalamus, third-order neurons enter the posterior third of the internal capsule, pass through the corona radiata, and terminate in the primary and secondary sensory areas of the parietal lobe cortex (areas 3,1, and 2). Notice that regardless of the level of entry into the spinal cord, pain and temperature stimulation delivered to one side of the body registers in the cerebral cortex of the opposite side.
Fast and Slow Pain
Pain sensation is often confusingly labeled "fast" or "slow" depending on the type of fiber which conducts the impulse and the speed with which the signal consciously registers. Fast pain, often called sharp or pricking pain, is usually conducted to the CNS over type A delta fibers. These ultimately excite lateral spinothalamic tract fibers which go directly to the VPL of the thalamus on the contralateral side. From here third-order fibers project to the cerebral cortex where they are somatotopically organized and sharply localized. Somatotopic organization means that each minute area of the sensory cortex receives input from a distinct peripheral area. A person can sharply localize a pain if he is able to tell exactly where it is originating. Slow pain, often called burning pain, is conducted to the CNS over smaller-diameter type C fibers. After entering the cord these fibers stimulate lateral spinothalamic tract neurons which send collaterals into the brainstem reticular formation. Fibers from the reticular formation diffusely project to the thalamus, hypothalamus, and possibly other areas as well, perhaps giving rise to the emotional component of pain. Pain signals following this route are poorly localized.
Dermatomes
A dermatome is the area of skin supplied by the afferent fibers in the posterior root of a single spinal nerve. Dermatomes tend to overlap each other so that stimulation of a specific point on the skin typically sends afferent signals into the cord over more than one posterior root. This is functionally important since destruction of a single posterior root does not totally eliminate sensation from the afflicted dermatome.
Touch and Pressure
Touch can be subjectively described as discriminating or crude. Discriminating (epicritic) touch implies an awareness of an object's shape, texture, three-dimensional qualities, and other fine points. Also implied here is the ability to recognize familiar objects simply by tactile manipulation. Crude (protopathic) touch, on the other hand, lacks the fine discrimination described above and doesn't generally give enough information to the brain to enable it to recognize a familiar object by touch alone. The tactile information implied here is of a much cruder nature than described for epicritic touch. The pathways to the brain for these two kinds of touch appear to be distinct.
Crude (Protopathic)
Touch and Pressure
General somatic mechanoreceptors sensitive to crude touch and pressure conduct information into the cord over GSA nerve fibers (Fig-2). The fibers pass up or down a few cord segments (neuromeres) in the dorsolateral (Lissauer) tract before synapsing chiefly in laminae VI, VII, and VIII. Second-order neurons cross over to the opposite side in the anterior white commissure to the anterior funiculus, where they turn upward in the anterior spinothalamic tract (ASTT) to the VPL of the thalamus. At higher pontine levels the tract also comes to lie close to the medial lemniscus as it ascends to the thalamus. Third-order neurons project from the VPL to areas 3, 1, and 2 of the cerebral cortex. Some of the ASTT fibers send collaterals into the brainstem reticular formation. While some of these no doubt ultimately reach the thalamus by reticulothalamic projections, the principal fate and function of these collaterals is largely unknown.
Fig-2

Discriminating (Epicritic) Touch, Pressure, and Kinesthesia
The conscious awareness of body position and movement is called the kinesthetic sense. It's important to recognize that there are many receptors throughout the body which continually conduct information to the brain concerning the body's position and movement and even the level of muscle tone. Such receptors are collectively called proprioceptors. However, not all of these signals reach the conscious level as a large portion are conducted instead to the brainstem and cerebellum for subconscious evaluation and integration. Only those proprioceptive signals reaching the conscious level contribute to the kinesthetic sense. The kinesthetic sense and discriminating touch and pressure pathways share a common route to the brain (Fig-3).
Fig-3

General somatic mechanoreceptors sensitive to discriminating touch and pressure and body position and movement conduct signals into the cord over GSA fibers. They pass directly into the ipsilateral posterior funiculus, where they turn upward in the dorsal columns to terminate in the dorsal column nuclei of the medulla. Those fibers entering the cord below the midthoracic level (i.e., from the lower trunk and legs) ascend through the medial dorsal column as the fasciculus gracilis and terminate in the nucleus gracilis. Fibers entering the cord above the midthoracic level (i.e., from the upper trunk and arms) enter the more lateral dorsal column and ascend as the fasciculus cuneatus to terminate in the more lateral dorsal column nuclei, the nucleus cuneatus. As might be expected, the dorsal columns include the fasciculus gracilis and fasciculus cuneatus while the dorsal column nuclei include the nucleus gracilis and nucleus cuneatus. Second-order neurons from these nuclei cross over to the other side of the brainstem in the lower medulla as the internal arcuate fibers. which then turn upward in the medial lemniscus to the VPL of the thalamus. Third-order neurons then project through the posterior limb of the internal capsule to areas 3, 1, and 2 of the cerebral cortex.
Much of the proprioceptive information which reaches the conscious level giving rise to the kinesthetic sense originates in joint receptors. However, recent evidence indicates that signals from muscle spindles may also represent a significant contribution to kinesthetic sensation. On the other hand, the subconscious proprioceptive information which is shunted to the brainstem and cerebellum for evaluation and integration arises chiefly in muscle spindles and Golgi tendon organs.
Subconscious Proprioception
Most of the subconscious proprioceptive input is shunted to the cerebellum. Further, signals arising in proprioceptors on the left side of the body register on the left side of the cerebellum. By contrast, sensory signals arising in the left side of the body register on the right side of the cerebral cortex. After entering the cord, proprioceptive afferents (GSA fibers) terminate in laminae V, VI, and VII (Clarke's column) of the posterior horn. Second-order neurons (primarily conducting information from Golgi tendon organs) cross over to the opposite side of the cord in the anterior white commissure to the lateral funiculus, where they turn upward in the anterior spinocerebellar tract (ASCT). After reaching upper pontine levels the fibers cross back over and enter the cerebellum through the superior cerebellar peduncle, where they terminate in the vermis (Fig-4). Some of the anterior spinocerebellar tract fibers upon reaching the medulla remain uncrossed and enter the cerebellum via the inferior cerebellar peduncle and terminate in the contralateral vermis. Other second-order neurons (those receiving information primarily from muscle spindles and tendon organs) leave Clarke's column to ascend in the ipsilateral posterior spinocerebellar tract (PSCT) to the cerebellum. After reaching the medulla, the fibers enter the cerebellum via the inferior cerebellar peduncle to terminate in the ipsilateral cortex.

Some of the subconscious proprioceptive input from the cervical region follows an alternate route to the cerebellum. Some of the fibers travel a short distance in the dorsal funiculus, terminating in the accessory cuneate nucleus of the medulla. Second-order neurons project from here as the cuneocerebellar tract to enter the cerebellum via the inferior cerebellar peduncle.
Posterior Funiculus Injury
Certain clinical signs are associated with injury to the dorsal columns. As might be expected, these are generally caused by impairment to the kinesthetic sense and discriminating touch and pressure pathways. They include (1) the inability to recognize limb position, (2) astereognosis, (3) loss of two-point discrimination, (4) loss of vibratory sense, and (5) a positive Romberg sign. Astereognosis is the inability to recognize familiar objects by touch alone. When asked to stand erect with feet together and eyes closed, a person with dorsal column damage may sway and fall. This is a positive Romberg sign.
GENERAL SOMATIC AFFERENT (GSA) PATHWAYS FROM THE FACE
Pain, Temperature, and Crude Touch and Pressure
General somatic nociceptors, thermoreceptors, and mechanoreceptors sensitive to crude touch and pressure from the face conduct signals to the brainstem over GSA fibers of cranial nerves V, VII, IX, and X. The afferent fibers involved are processes of monopolar neurons with cell bodies in the semilunar, geniculate, petrosal, and nodose ganglia, respectively. The central processes of these neurons enter the spinal tract of V, where they descend through the brainstem for a short distance before terminating in the spinal nucleus of V. Second-order neurons then cross over the opposite side of the brainstem at various levels to enter the ventral trigeminothalamic tract, where they ascend to the VPM of the thalamus. Finally, third-order neurons project to the "face" area of the cerebral cortex in areas 3, 1, and 2 (Fig-5).
Fig-5

Discriminating Touch and Pressure
The pathway for discriminating touch from the face is illustrated in Fig-6. Signals are conducted from general somatic mechanoreceptors over GSA fibers of the trigeminal nerve into the principal sensory nucleus of V, located in the middle pons. Second-order neurons then conduct the signals to the opposite side of the brainstem, where they ascend in the medial lemniscus to the VPM of the thalamus. Thalamic neurons then project to the "face" region of areas 3, I, and 2 of the cerebral cortex.
Fig-6

Kinesthesia and Subconscious Proprioception
Proprioceptive input from the face is primarily conducted over GSA fibers of the trigeminal nerve. Curiously, however, the cell bodies of these monopolar neurons are located in the mesencephalic nucleus of V in the midbrain rather than the semilunar ganglia, where the cell bodies of other afferent neurons of the trigeminal nerve are located. The peripheral endings of these neurons are the general somatic mechanoreceptors sensitive to both conscious (kinesthetic) and subconscious proprioceptive input. Their central processes extend from the mesencephalic nucleus to the principal sensory nucleus of V in the pons (Fig-7).
Fig-7

The subconscious component is conducted to the cerebellum, while the conscious component travels to the cerebral cortex. Certain second-order neurons from the principal sensory nucleus relay proprioceptive information concerning subconscious evaluation and integration into the ipsilateral cerebellum. Other second-order neurons project to the opposite side of the pons and ascend to the VPM of the thalamus as the dorsal trigeminothalamic tract. Thalamic projections terminate in the face area of the cerebral cortex.
SPECIAL SOMATIC AFFERENT (SSA) PATHWAYS
Hearing
The organ of Corti with its sound-sensitive hair cells and basilar membrane are important parts of the sound transducing system for hearing. Mechanical vibrations of the basilar membrane generate membrane potentials in the hair cells which produce impulse patterns in the cochlear portion of the vestibulocochlear nerve (VIII). The principles of this system will be examined elsewhere. For now we will examine only the central pathways from the receptors to their terminations in the brain (Fig-8).
Special somatic nerve fibers of cranial nerve VIII relay impulses from the sound receptors (hair cells) in the cochlear nuclei of the brainstem. These are bipolar neurons with cell bodies located in the spiral ganglia of the cochlea. Their central processes terminate in the dorsal and ventral cochlear nuclei on the ipsilateral side of the brain stem at the pontomedullary border. Most of the second-order neurons arising in the cochlear nuclei cross to the opposite side of the brainstem in the trapezoid body and turn upward in the lateral lemniscus, terminating in the inferior colliculus of the midbrain. Collaterals of the lateral lemniscus terminate in the nucleus of the trapezoid body, superior olivary nucleus, nucleus of the lateral lemniscus, and the brainstem reticular formation. Fibers arising in these nuclei also ascend in the lateral lemniscus. Those fibers from the cochlear nuclei which don't cross over in the trapezoid body ascend in the ipsilateral lateral lemniscus to the inferior colliculus. Sound signals also pass from one side to the other via contralateral projections from one lemniscal nucleus to the other as well as from one inferior colliculus to the other. Thus each lateral lemniscus conducts information from both sides, which helps to explain why damage to a lateral lemniscus produces no appreciable hearing loss other than problems with sound localization. Signals are then conducted from the inferior colliculi to the medial geniculate bodies and finally to the primary auditory area of the temporal lobes (area 41).
Fig-8

Vestibular System
The vestibulocochlear nerve serves two quite different functions. The cochlear portion, previously described, conducts sound information to the brain, while the vestibular portion conducts proprioceptive information. It is the central neural pathways of the latter function which we will examine now (Fig-9). The mechanics and physiology of the vestibular system explained here.
VESTIBULAR SYSTEM
The vestibulocochlear nerve (VIII) has the dual function of serving both the sense of hearing (via cochlear fibers) and proprioception (via vestibular fibers).
THE VESTIBULAR APPARATUS
A cavernous network called the bony labyrinth exists within the temporal bone on either side of the head. Within this bony labyrinth is a membranous labyrinth of roughly the same shape filled with endolymph, the same fluid present in the cochlear duct of the inner ear (Fig-1). The endolymph in both the vestibular and cochlear systems is continuous, and is formed in the endolymphatic sac, which makes contact with the fluid of the temporal dura. The space between the membranous and bony labyrinths is filled with perilymph.
The membranous labyrinth is composed of three semicircular canals. Each canal is twice connected to the utriculus, a large endolymph-containing sac. The endolymph of each canal is continuous with that in the utriculus at one end, and separated from it at the other end by a flexible mechanosensitive barrier called the crista ampullaris. The crista is located in the enlarged end of each canal known as the ampulla. The anterior and posterior canals are essentially vertical when a person holds his head erect and they are at right angles to each other. The lateral canal is almost horizontal (actually elevated 23° anteriorly) and forms a plane at right angles to the other two. This geometric arrangement provides the vestibular system with the capability of detecting movements of the head in all directions.
The utriculus is continuous with a second endolymphatic enlargement, the sacculus. A mechanosensitive structure, the macula acustica, is located in the wall of the utriculus with a second macula located in the saccular wall. The three cristae and two maculae are the actual proprioceptive units in each vestibular apparatus. The cristae and maculae are in neural contact with the central nervous system through SSA VIII nerve fibers. Mechanosensitive hair cells in the cristae and maculae form two-element receptors with these fibers.
Fig-1

Figure-2 illustrates the distribution of the vestibular nerve fibers to the membranous labyrinth. Notice that one branch of the nerve is distributed to each ampulla, where it distributes to the crista ampullaris hair cells. Separate branches of the nerve are also distributed to the maculae of the utriculus and sacculus, where they form two-element receptors with the macular hair cells.
Fig-2

The Crista Ampullaris
Fig-3

The crista ampullaris is a mechanosensitive flexible barrier to the flow of endolymph between one end of the semicircular canal and the utriculus (Fig-3). A number of sensitive hair cells are interposed with supporting cells at the base of the crista within the ampulla. The hair cell hairs project into a gelatinous mass, the cupola, which projects upward to form a flexible barrier across the space of the ampulla. The cupola behaves like an elastic diaphragm rather than like a swinging door. Angular movements of the head cause the endolymph to push against the cupola so that it bows in one direction or the other. Deflection toward the utriculus is utriculopetal deflection, while deflection away from the utriculus is utriculofugal deflection. Deflecting the cupola bends the hairs, excites the hair cells, and produces impulses in the SSA VIII nerve fibers. In this way the CNS is informed of movements of the head.
There are two types of hair cells in the vestibular apparatus. Type I hair cells are somewhat spherical in shape with 60 to 70 small hairs (stereocilia) emerging from the cuticle (Fig-4). A particularly long hair process, the kinocilium, stands at one end of the stereocilia. Type II hair cells are more cylindrical in shape but their stereocilia and kinocilia are identical with type I cells.
Fig-4

SSA VIII nerve fibers are in close contact with both types of cells, although they form more extensive processes around the base of type I cells. In addition to the SSA fibers, there is evidence that small-diameter efferent fibers of unknown origin also innervate the hair cells. They form direct synaptic contacts with the type II cells but appear instead to terminate on SSA fibers of the type I cells. The origin and function of these efferent fibers is unknown. It seems likely that they may in some way influence the excitability of the hair cells and their potential for producing impulses in the SSA VIII nerve fibers.
Hair Cell Stimulation and Cochlear Nerve Discharge
The stereocilia and kinocilium of each hair cell project up into the gelatinous cupola. Consequently, whenever the cupola is displaced, either toward the utriculus or away from it, the hairs are also deflected. Deflection of the hairs toward the kinocilium produces a change in the hair cell sufficient to increase the firing rate in the SSA VIII nerve fibers. Conversely, deflection away from the kinocilium decreases the firing rate (Fig-5).
Fig-5

The hair cells in a given crista ampullaris are all orientated in the same direction so that deflection of the cupola either bends all the hairs toward the kinocilia or away from it. Thus deflection of the cupola either increases or decreases the firing rate of the SSA VIII nerve fibers.
In the lateral canals, the kinocilia all face the utriculus. In the vertical canals they all face away from the utriculus, toward the canal. Thus, utriculopetal deflection in the lateral canals produces an increase in the firing rate, while utriculofugal deflection produces a decrease. However, just the opposite is true concerning the vertical canals. Here the hair cell kinocilia are oriented in the opposite direction so that utriculopetal deflection causes a decrease while utriculofugal deflection produces an increase in the firing rate.
Coplanar Canals are Functional Units
The anterior canal on one side of the head and the posterior canal on the opposite side are in the same plane. Thus the two canals are a functional unit since any head movement which causes utriculofugal deflection in the anterior canal on one side will be matched by utriculopetal deflection in the posterior canal on the opposite side (Fig-6). A similar relationship exists with the two lateral canals and they also form a functional unit (Fig-7).
Fig-6

Fig-7

Hair cells stimulate SSA VIII nerve fibers via chemical synapses. Because a fairly steady resting discharge of 40 to 60 impulses per second can be recorded in the nerve fibers, it is assumed that a small amount of transmitter chemical (possibly a catecholamine) is constantly being released. It has been proposed that displacement of the hairs toward the kinocilium increases the firing rate by increasing the rate or amount of transmitter released by the hair cell. Likewise, displacement of the hairs in the opposite direction decreases the firing rate by lowering the rate or amount of release.
In contrast to the stereocilia, which are embedded in the cuticle, the base of the kinocilium is in direct contact with the hair cell cytoplasm. The kinocilium plunging inward (with the aid of the stereocilia leaning against it) may depolarize the hair cell membrane and establish a receptor potential, which in turn causes transmitter release. Alternatively, deflection of the stereocilia away from the kinocilium pulls the kinocilium outward, hyperpolarizing the membrane and decreasing transmitter release.
The cristae are particularly sensitive to changes in angular acceleration and deceleration of the head. The greatest change in firing rate along nerve fibers from the cristae occur at the beginning and end of angular movements of the head. As Fig-7 shows, the inertia of the endolymph when the head first starts rotating to the left produces utriculopetal deflection in the left canal and utriculofugal deflection in the right canal. Thus we see a large initial change in firing rate from each canal at the beginning of the movement. However, if the rotation of the head to the left continues, we see no further change in firing rates until the rotation begins to slow down (decelerate). At this point. the inertia of the endolymph causes the cupola to deflect in the opposite direction, once again causing a change in the firing rate. This time, however, there is a decrease in the left canal and an increase in the right canal. Thus one can see that the canal system is particularly adept at signaling changes in acceleration and deceleration of the head's angular movements. Further, because the canals are arranged in three planes, angular movements in all directions are easily detected by the canal system. No doubt angular movements which are not exactly parallel with a single coplanar canal system are detected by the brain through some "weighted" input from two or more coplanar functional units.
The Macula Acustica
The macula acustica is a mechanosensitive structure in the utriculus and sacculus. It is similar to the crista in that the base of the structure is composed of type I and type II hair cells (Fig-8). Likewise, the base of the hair cells forms contacts with SSA VIII nerve fibers. Maculae are also called otolith receptors because the hair processes project into a low-lying gelatinous structure which is impregnated with dense calcareous formations called otoliths or otoconia. The otolith receptors respond to static gravitational pull and are therefore well equipped to signal the position of the head in space at any given time. A basal discharge rate of the SSA VIII fibers from the utriculus is observed when the head is in the normal erect position. This rate increases to a maximum when the head is moved to a position 90° from normal (i.e., 90° forward, backward, or to either side).
In addition to their gravitational or static response, utricular otolith receptors also respond to linear acceleration and deceleration of the head, thus exhibiting a dynamic response characteristic as well. Saccular otolith receptors respond only to the static position of the head in space and demonstrate no appreciable dynamic response.

Fig-8
VESTIBULAR SYSTEM INTERACTIONS
Vestibular Control of Eye Movements
An interesting cooperative relationship exists between the vestibular system and the extraocular muscles of the eye. Those eye movements caused by vestibular stimulation are generally compensatory in nature, attempting to keep to the visual axis relatively fixed when the head is moved in space. This aids both vision and the maintenance of posture. As an example, a cooperative relationship exists between the lateral canals on both sides of the head that is designed to keep the eyes directed toward a reference point in the visual field as the head is moved in a lateral plane (Fig-9). Unless consciously overridden, the eyes move slowly to the left as the head is turned slowly to the right, maintaining a constant reference point in the visual field.
These reflex conjugate eye movements are produced by changes in activity of the extraocular eye muscles in response to vestibular activity. A close examination of Fig-9 shows that when the head is turned to the right, the endolymph in the right lateral canal deflects the cupola toward the utriculus (utriculopetal), while the endolymph of the left lateral canal deflects its cupola away from the utriculus (utriculofugal). Now if we remember that utriculopetal deflection in the lateral canals increases the firing rate while utriculofugal deflection decreases it, an examination of the neural circuitry in Fig-9 explains the slow shift of the eyes to the left. The lateral rectus muscle of the left eye and medial rectus of the right eye both contract, while their antagonists relax, pulling the eyes slowly to the left. A similar cooperative relationship exists between the anterior canal on one side and the posterior canal on the other. The anterior canals are able to produce stimulation of the ipsilateral superior rectus muscle and the contralateral inferior oblique. The posterior canals produce stimulation of the ipsilateral superior oblique and the contralateral inferior rectus muscle. In this way the eyes can maintain their reference point when the head is moved through any plane.
The Vestibulospinal System
While the vestibular system responds primarily to movements of the head, it is able to produce far-reaching postural changes throughout the body. The vestibular system can regulate alpha and gamma motor neuron activity in the spinal cord through the lateral and medial vestibulospinal tracts (Fig-7). The vestibulospinal tracts originate in the vestibular nuclei of the brainstem. Those fibers which originate in the lateral vestibular (Deiter's) nucleus descend ipsilaterally in the anterior funiculus and form the lateral vestibulospinal tract. The fibers of this tract terminate in laminae VII, VIII, and IX at all levels of the cord. Arising from the medial vestibular nucleus are the fibers of the medial vestibulospinal tract. While there is a small crossed component, most of its fibers descend ipsilaterally only as far as the midthoracic level, where they too synapse in laminae VII, VIII, and IX.
The vestibulospinal tracts facilitate extensor and inhibit flexor alpha and gamma motor neurons. Input to the vestibular nuclei via fibers of cranial nerve VIII from the vestibular apparatus presupposes an antigravity or postural role for the vestibulospinal tracts. Activity in these tracts is also influenced by input to the vestibular nuclei from the cerebellum, and through it, the peripheral proprioceptors of muscles, tendons, and joints.
The Vestibular System and the Cerebellum
Because of the role the vestibular system plays in the maintenance of posture and muscle control, it is not surprising to find that the system has a close relationship with the cerebellum. Both first- and second-order vestibulocerebellar fibers end as mossy fibers on the granular cells of the cerebellar cortex of the flocculonodular lobe. In addition, the fastigial and dentate cerebellar nuclei also receive vestibular input. Presumably the cerebellar cortex integrates the vestibular input with other proprioceptive input from all parts of the body. The cerebellum is then in a position to exert influence on the postural musculature via output to the vestibular, reticular, and red nuclei. Vestibulospinal, reticulospinal, and rubrospinal fibers influence muscle activity at the spinal cord level, while cerebellar output through the thalamus to the cerebral cortex modifies motor activity at the cortical source.
Vestibulocortical Projections
In order to be consciously aware of position and movements of the head in space, it is necessary that vestibular information reach the cerebral cortex. The kinesthetic sense (conscious awareness of body position and movement) requires cortical input from peripheral proprioceptors as well as from the vestibular system. The cortical area which receives this information is located in the postcentral gyrus near the somatosensory projection of the mouth. Vestibulocortical projections appear to be primarily contralateral with intermediate synapses in the ipsilateral vestibular nuclei and the contralateral thalamus.
Vestibular System and Autonomic Effects
The effects of vestibular activity on autonomic function are well known and are grouped under the heading "motion sickness." They include effects on the vasomotor system (typically a vasodepressor action with a blood pressure drop), an increase in the rate and depth of respiration, decreased salivation, increased sweating, pupillary dilation, and disturbances of the gastrointestinal tract. Most of these effects are mediated through the sympathetic nervous system.
Tests for the Integrity of the Semicircular Canals
Certain bodily responses to vestibular stimulation are reflexly predictable, such as conjugate movements of the eyes and other postural adjustments of the body. The integrity of the various canals can be tested by their capacity to produce the expected responses. The rotation (swivel chair) test and the caloric test are both designed to do this.
The rotation test allows maximum stimulation of the horizontal and vertical canals. Maximum deflection of the cupola of a particular canal occurs when the movement of the head is in the same plane as the canal which contains that cupola. This is accomplished in the swivel chair by placing the head in various positions and then rotating the chair. Recall that maximum deflection in a canal on one side of the head is accompanied by maximum deflection in its functional counterpart on the opposite side.
Predictable responses observed with rotation tests are nystagmus, vertigo, and past pointing. Nystagmus refers to rapid to-and-fro movements of the eyes. As previously noted, the eyes slowly shift to the left as the head is turned slowly to the right. Of course there is a limit to how far left the eyes can shift if the head continues turning to the right. When they have pulled as far left as possible, they suddenly "snap" back to the right and "fix" on a new reference point in the visual field. This alternating slow phase to the left followed by a fast phase to the right continues as the head keeps rotating to the right unless consciously overridden. While nystagmus technically refers to the eye shifts in both directions, neuroscientists typically refer to nystagmus as the direction of the fast phase. For example, nystagmus is to the right in the case just described.
Because cupola deflection directly controls eye movements, and because this deflection is in one direction during the acceleration phase of the angular rotation and in the opposite direction during the deceleration phase, it follows that nystagmus is in one direction during rotation (perrotation) and in the opposite direction after rotation (postrotation). Perrotational nystagmus is in the same direction as the rotation. However, if the rotating chair is suddenly stopped, the canals cease to rotate but the inertia of the endolymph is not so easily overcome. Consequently the cupolae are deflected in the opposite direction for a brief period of time, producing a postrotational nystagmus in the direction opposite the rotation.
Vertigo and past pointing are also predictably observed following rotation in a normal individual. Vertigo is the sensation of a movement when no such movement exists. This is caused by the fact that once the actual turning stops, the inertia of the endolymph remains for a while, deflecting the cupolae and sending signals to the brain that turning is still occurring. Normally the vertigo (false sense of movement) is in the same direction as the postrotational nystagmus. The body will ordinarily attempt to reflexly make postural adjustments for the vertigo just as it would for a real movement. Thus, predictable leaning of the whole body (a reflex attempt to correct for the false movement) is typically observed following a period of rotation. Specifically, the body leans in the direction opposite the postrotational nystagmus, An extended arm also points in the direction opposite the post rotational nystagmus. This is past pointing,
The rotation test has the disadvantage of not allowing the canals on each side of the head to be tested separately. However, caloric tests, which involve the introduction of hot or cold solutions into the auditory canal, allow the clinician to test each side of the head separately. A hot water solution introduced into the auditory canal causes the endolymph to expand, deflecting the cupola in a predictable direction. This is later followed by the use of a cold water solution which cools the endolymph, producing deflection in the opposite direction. Like the rotation test, predictable changes in nystagmus, vertigo, and past pointing can be observed.
Vestibular System
The vestibulocochlear nerve serves two quite different functions. The cochlear portion, previously described, conducts sound information to the brain, while the vestibular portion conducts proprioceptive information. It is the central neural pathways of the latter function which we will examine now (Fig-9). The mechanics and physiology of the system explained elsewhere.
Fig-9

Special somatic afferent fibers from the hair cells of the macula utriculi and macula sacculi conduct information into the vestibular nuclei on the ipsilateral side of the pons and medulla. These are bipolar neurons with cell bodies located in the vestibular ganglion. Some of the fibers project directly into the ipsilateral cerebellum to terminate in the uvula, flocculus, and nodulus, but most enter the vestibular nuclei and synapse there.
As might be expected, neuronal output from the vestibular nuclei effects bodily and eye movements in response to movements of the head as detected by the vestibular apparatus. The vestibulospinal path fibers which affect body reflexes and muscle tone in response to vestibular input originate primarily in the lateral vestibular nucleus. The medial vestibular nucleus is the principal origin of both crossed and uncrossed fibers which descend through the brain stem in the medial longitudinal fasciculus to the upper cord causing various reflex head and arm movements in response to vestibular stimuli. Finally, all four vestibular nuclei (medial, lateral, superior, and inferior) project both crossed and uncrossed fibers to the motor nuclei of cranial nerves Ill, IV, and VI in order to control and coordinate reflex eye movements. These vestibuloocular paths also travel in the medial longitudinal fasciculus.
Vision
The visual system receptors are the rods and cones of the retina. The neurophysiology of vision and visual reflexes are discussed elsewhere. Special somatic afferent fibers of the optic nerve (II) conduct visual signals into the brain. Examination of Fig-10 will show that fibers from the lateral (temporal) retina of either eye terminate in the lateral geniculate body on the same side of the brain as that eye. On the other hand, SSA II fibers from the medial (nasal) retina of each eye cross over in the optic chiasm to terminate in the contralateral lateral geniculate body. The optic nerve is composed of fibers from the retina to the optic chiasm. Even though no synapses occur in the optic chiasm, the continuation of the visual pathway from the optic chiasm to the lateral geniculate body is called the optic tract rather than the optic nerve. After a synapse in the lateral geniculate body, the signal continues in the optic radiation to area 17 of the conscious visual cortex. Area 17 is the primary visual area, which receives initial visual signals. Neurons from this area project into the adjacent occipital cortex (areas 18 and 19) which is known as the secondary visual area. It is here that the visual signal is fully evaluated.
Fig-10

Fig-11

The visual reflex pathway involving the pupillary light reflex is illustrated in Fig-11. This is the well-known reflex in which the pupils constrict when a light is shined into the eyes and dilate when the light is removed. Some SSA II fibers leave the optic tract before reaching the lateral geniculates, terminating in the superior colliculi instead. From here, short neurons project to the EdingerWestphal nucleus (an accessory nucleus of III) in the midbrain, which serves as the origin of the preganglionic parasympathetic fibers of the oculomotor nerve (GVE III). The GVE III fibers in turn project to the ciliary ganglia, from which arise the postganglionic fibers to the sphincter muscles of the iris, which constrict the pupils.
GENERAL VISCERAL AFFERENT (GVA) PATHWAYS
Pain and Pressure Sensation via the Spinal Cord
Visceral pain receptors are located in peritoneal surfaces, pleural membranes, the dura mater, walls of arteries, and the walls of the GI tube. Nociceptors in the walls of the GI tube are particularly sensitive to stretch and overdistension.
General visceral nociceptors conduct signals into the spinal cord over the monopolar neurons of the posterior root ganglia. They terminate in laminae III and IV of the posterior horn as do the pain and temperature pathways of the GSA system; however, their peripheral processes reach the visceral receptors via the gray rami communicantes and ganglia of the sympathetic chain (Fig-12), Second-order neurons from the posterior horn cross in the anterior white commissure and ascend to the thalamus in the anterior and lateral spinothalamic tracts, Projections from the VPL of the thalamus relay signals to the sensory cortex.
Fig-12

Fig-13

The localization of visceral pain is relatively poor, making it difficult to tell the exact source of the stimuli. At least a partial explanation of our inability to precisely localize visceral pain relates to its rarity. True visceral pain seldom occurs when compared to the frequency of external pain. An additional compounding factor is the phenomena of referred pain. Because true visceral pain is often projected or "referred" by the brain to some area on the surface of the body, its true visceral origin is often confused. The mechanism for referred visceral pain is not fully understood but may result in part from the close proximity in the posterior horn of the central terminals of GVA pain fibers and GSA spinal nerve fibers from the body surface. This is supported by the fact that pain from a visceral origin is referred to a dermatome with which it shares the same posterior root. This is a useful observation, often making it possible to locate the source of a visceral pain from an observation of the surface area to which it is referred. The pain down the inside of the left arm associated with true cardiac pain is a good example.
It is likely that separate second-order neurons relay pain information from GSA and GVA input. If the painful stimulus to the viscera is moderate, the level of activity in the GVA fibers is likely sufficient to stimulate only those second-order neurons which normally relay signals from the viscera. However, if the painful stimulus increases in strength, the increased central synaptic activity of the GVA neurons may "spill over" and raise the central excitatory state of those second-order neurons which normally relay information from GSA fibers of the dermatome. If the painful visceral stimulation is very strong, this "spill over" may be sufficient to exceed the threshold of excitation for these neurons, causing them to fire even though no painful stimulus is delivered to the general somatic nociceptors of the dermatome. Thus the brain incorrectly projects the source of the pain to the dermatomal area (Fig-13).
Blood Pressure, Blood Chemistry, and Alveolar Stretch Detection
The walls of the aorta and the carotid sinuses contain special baroreceptors (pressure receptors) which respond to changes in blood pressure. These mechanoreceptors are the peripheral endings of GVA fibers of the glossopharyngeal (IX) and vagus (X) nerves. The GVA fibers from the carotid sinus baroreceptors enter the solitary tract of the brainstem and terminate in the vasomotor center of the medulla (Fig-14). This is the CNS control center for cardiovascular activity. The cell bodies of these unipolar neurons are located in the petrosal ganglion. GVA fibers of the vagus nerve conduct signals from the baroreceptors in the walls of the aorta to the solitary tract and on to the vasomotor center. The cell bodies of these unipolar neurons are located in the nodose ganglion.
Fig-14

Stretch receptors in the alveoli of the lungs conduct information concerning rhythmic alveolar inflation and deflation over GVA X fibers to the solitary tract and then to the respiratory center of the brainstem. This route is an important link in the Hering-Breuer reflex, which helps to regulate respiration.
Carotid body chemoreceptors, sensitive to changes in blood PO2 and, to a lesser extent, PCO2 and pH, conduct signals to both the vasomotor and respiratory centers over GVA IX nerve fibers. GVA X fibers conduct similar information from the aortic chemoreceptors to both centers. Chemoreceptors were discussed elsewhere.
SPECIAL VISCERAL AFFERENT (SVA) PATHWAYS
Taste
The receptors for taste are the taste cells which produce impulses in afferent fibers in response to chemical stimulation. They were described elsewhere. The pathways for taste sensation are illustrated in Fig-15. Special visceral afferent (SVA) fibers of cranial nerves VII, IX, and X conduct signals into the solitary tract of the brainstem, ultimately terminating in the nucleus of the solitary tract on the ipsilateral side. Second-order neurons cross over and ascend through the brainstem in the medial lemniscus to the VPM of the thalamus. Thalamic projections to area 43 (the primary taste area) of the postcentral gyrus complete the relay. SVA VII fibers conduct from the chemoreceptors of taste buds on the anterior twothirds of the tongue, while SVA IX fibers conduct taste information from buds on the posterior one-third of the tongue. SVA X fibers conduct taste signals from those taste cells located throughout the fauces.
Fig-15

Fig-16

Smell
The sense of smell was examined elsewhere and, once again, we will look only at the central pathways here. The smell-sensitive cells (olfactory cells) of the olfactory epithelium project their central processes through the cribiform plate of the ethmoid bone, where they synapse with mitral cells. The central processes of the mitral cells pass from the olfactory bulb through the olfactory tract, which divides into a medial and lateral portion (Fig-16). The lateral olfactory tract terminates in the prepyriform cortex and parts of the amygdala of the temporal lobe. These areas represent the primary olfactory cortex. Fibers then project from here to area 28, the secondary olfactory area, for sensory evaluation. The medial olfactory tract projects to the anterior perforated substance, the septum pellucidum, the subcallosal area, and even the contralateral olfactory tract. Both the medial and lateral olfactory tracts contribute to the visceral reflex pathways, causing the viscerosomatic and viscerovisceral responses described earlier.
DAMAGE TO THE SPINAL NERVES AND SPINAL CORD
After studying the motor pathways and the sensory pathways, the injuries described in Table-1 would be expected to produce the symptoms listed.
Table-1 Symptoms of Damage to Spinal Nerves and Spinal Cord
Damage Possible cause of damage Symptoms associated with innervated area
1.Peripheral nerve Mechanical injury Loss of muscle tone. Loss of reflexes. Flaccid paralysis. Denervation atrophy. Loss of sensation.
2.Posterior root Tabes dorsalis Paresthesia. Intermittent sharp pains. Decreased sensitivity to pain. Loss of reflexes. Loss of sensation. Positive Romberg sign. High stepping and slapping of feet.
3.Anterior Horn Poliomyelitis Loss of muscle tone. Loss of reflexes. Flaccid paralysis. Denervation atrophy.
4.Lamina X (gray matter) Syringomyelia Bilateral loss of pain and temperature sense only at afflicted cord level. Sensory dissociation. No sensory impairment below afflicted level.
5.Anterior horn and lateral corticospinal tract Amyotrophic lateral sclerosis Muscle weakness. Muscle atrophy. Fasciculations of hand and arm muscles. Spastic paralysis.
6.Posterior and lateral funiculi Subacute combined degeneration. Loss of position sense. Loss of vibratory sense. Positive Romberg sign. Muscle weakness. Spasticity. Hyperactive tendon reflexes. Positive Babinski sign.
7.Hemisection of the spinal cord Mechanical injury Brown-Sequard syndrome.
Below cord level on injured side.
Flaccid paralysis. Hyperactive tendon reflexes. Loss of position sense. Loss of vibratory sense. Tactile impairment.
Below cord level on opposite side beginning one or two segments below injury.
Loss of pain and temperature.

No comments:
Post a Comment