
NKCP, a purified filtrate of Bacillus subtilis var. natto culture is a food based extract of “natto”, a Japanese traditional fermented food made from soybean. Purification to remove most of distinctive odor of natto and its vitamin K2 yields an easy-to-eat food that has a wide variety of uses as a functional food. NKCP contains proteolytic enzymes secreted by Bacillus subtilis var. natto (Bacillus subtilis var. natto-produced protein), which balance clotting mechanisms in the blood.
In vitro and clinical studies have demonstrated that the consistent intake of NKCP over a prolonged period helps to maintain normal circulation. The safety of NKCP has been demonstrated in safety studies. *In vitro (Latin for "within the glass") refers to the technique of performing a given procedure in a controlled environment outside of a living organism.
Patents (Production process for purified filtrate of Bacillus subtilis var. natto culture)
Japan (No.3532503)
NKCP stands for Purified Natto Culture Filtrate. NKCP tablets launched April 2001. Daiwan Health Development Inc. (DHD) began export of NKCP on November 2001.
Development Background
The “People's Health Promotion Campaign for the 21st Century (Healthy Japan 21)” was launched in 2000 by the Ministry of Health, Labour and Welfare. The purposes of this campaign are to reduce premature death, prolong optimal health, and improve the quality of life. Simply put, it focuses on longevity accompanied by optimal health. Cardiovascular and cerebrovascular diseases account for about 30% of causes of death in Japan, about 25% in the world. To lower mortality rates while in the prime of life, it is very important to prevent cardiac and cerebrovascular diseases.
A patient with cardiovascular disease may evade a fatal cardiovascular event, but eventually their quality and duration of life will be compromised.
Contemporary lifestyles choices are associated with an increased risk of thrombus formation and subsequent ischemic heart disease and cerebrovascular disease. It has recently been demonstrated that the etiology of travel induced thrombosis, also known as “economy class syndrome”, is Deep Vein Thrombosis (DVT), which may result in a pulmonary embolism. DVT may develop as a result of prolonged sitting in cramped quarters related to both air and auto travel. Additionally, common ailments such as stiff shoulder muscles and leg edema may be caused by insufficient peripheral circulation, caused by excess blood viscosity. The key to preventing DVT and insufficient peripheral circulation is to balance coagulation and fibrinolysis, to prevent thrombus formation and to enhance overall cardiovascular health and longevity. NKCP may be a critical component to achieve such balance.
Development
NKCP was developed based on the traditional Japanese food, natto. Natto contains constituents that enhance the fibrinolysis system in favor of clot lysis. Naturally occurring proteolytic enzymes produced by Bacillus subtilis var. natto dissolve clots in a balanced manner, without causing excessive blood thinning. Recent research has shown that other additional constituents from Bacillus subtilis var. natto produce a substance that acts to inhibit blood coagulation, thereby complementing the known fibrinolytic activity to improve blood viscosity. Based on these insights, it is probable that consistent consumption of natto may lower the overall risk of thrombus formation. However, compliance of such a recommendation may be poor, due to natto's potentially objectionable odor and flavor. Additionally, natto consumption may be contraindicated for patients on anti-coagulant therapy due to its high vitamin K2 content. Furthermore, the proteolytic enzyme content may vary greatly in commercially available natto, resulting in inconsistent anti-coagulant activity.
NKCP was therefore developed to provide a raw material for food products which corrects the drawbacks of natto. NKCP is produced by fermenting the bacillus in a liquid medium containing soybean extract and then partially purifying the peptidase. The odor, bacterial body and vitamin K2 content are reduced to a negligible level. NKCP is designed to contain a constant amount of peptidase.
Characteristics of NKCP
• NKCP is extracted from Bacillus subtilis var. natto and it is free of the undesirable odor and viscous texture of natto.
• NKCP's purpose is three-fold.
To function as (1) an anticoagulant (2) thrombolytic and (3) decreases blood viscosity.
• The majority of the vitamin K2 has been eliminated, therefore it is less antagonistic to other drugs such as warfarin.
• NKCP is standardized to contain specific levels of proteolytic enzymes secreted by Bacillus subtilis var. natto.
• The principal functional enzyme (protease) is stable at pH 6.0-10.0 and at temperatures 60℃ or below.
• The safety of NKCP has been confirmed in many animal and human studies.
• The production process for this purified filtrate of Bacillus subtilis var. natto culture is registered under Patent No. 3532503 in Japan.
Suggested daily dose
125-500mg/day
Mechanism of Action
NKCP is shown to have the following effects:
a. Inhibiting thrombus formation in vitro and in vivo.
b. Decreasing the viscosity of blood in vitro and in vivo.
c. Lysing thrombi in vitro and in vivo.
*In vivo (of processes) performed or taking place in a living organism."fluid transport was measured in vivo"
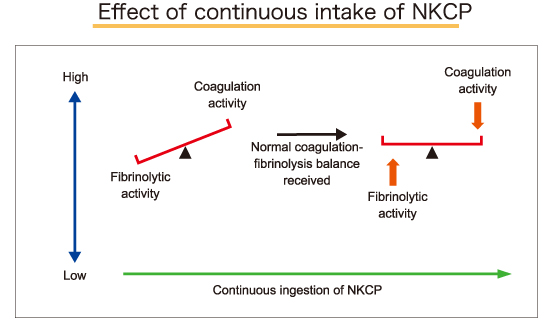
The coagulation/fibrinolysis system is comprised of a series of complicated reactions designed to maintain the balance between healthy circulation and prevention of excess bleeding. Many factors can influence this system, however, it is not easily disrupted. In the event that the system shifts towards excess thrombus formation, it is challenging to return the system back to balance. Because it is difficult to lyse a formed thrombus, the emphasis should be placed on prevention of thrombus formation rather than on thrombolysis.
By inhibiting thrombus formation and decreasing blood viscosity, orally administered NKCP helps maintain balance, shifts blood away from clot formation, and enhances circulation throughout the body.
Scientific Data
1. NKCP Actions
Reduced risk of thrombus formation requires: 1) clot formation prevention 2) maintenance of normal blood viscosity and 3) lysing blood clots (thrombi).
NKCP, derived from Bacillus subtilis var. natto, has been shown to perform these three functions.
2. Supporting Scientific Data
(1) Anticoagulant effect
▶Anticoagulant effect of NKCP in human blood.
▶Anticoagulant effect of NKCP in rat model of thrombosis formation.
(2) Action of preventing increase in blood viscosity.
▶Effect of NKCP on the viscosity of human blood
(3) Thrombolytic effect.
▶Thrombolytic effect of NKCP on artificial thrombi.
▶In vivo thrombolytic effect of oral NKCP in experimental thrombolysis model.
▶Identification of proteases derived from Bacillus subtilis var. natto related to thrombolysis.
3. Clinical Study Results
▶Effect of Bacillus subtilis var. natto-derived protein on the human blood coagulation/fibrinolysis system.
Safety
Single-dose:
LD50>5,000mg/kg
Repeated-dose:
NOAEL
Males: > 1,325mg/kg body weight/day.
Females: > 1,541mg/kg body weight/day.
Mutagenicity:
Negative (± metabolic activation).
Antigenicity (guinea pigs): Negative for active systemic anaphylactic reaction (ASA) and passive cutaneous anaphylactic reaction (PCA).
Effect on bleeding time (rats):
In rats orally given NKCP, a 0.5mm incision was made in the tail tip after 1 hour to measure bleeding time. NKCP at 300mg/kg did not prolong the bleeding time.
Interaction with warfarin (rats):
NKCP at 250mg/kg was administered into the duodenum by the in situ loop method in rats, in which bleeding time was delayed by treatment with warfarin, and blood collected after 6 hours was measured for coagulation time. The warfarin treatment significantly prolonged the coagulation time in comparison with the control group, but no added delay of coagulation was observed in the warfarin + NKCP treatment group compared with warfarin treatment group.
Long-term administration (humans):
Twenty-three healthy adults were given NKCP at 250mg/day for 12 weeks, and no clinically significant adverse events were observed. There were no statistically significant changes in hematological or biochemistry tests.
Five healthy adults were given NKCP at 750mg/day for 6 consecutive weeks to study and observe changes in laboratory test values (hematological tests, biochemistry tests, and blood coagulation/fibrinolysis parameters) and adverse events. As a result, ELT shortened, t-PA decreased, and thromboplastinogen activity test (TAT) increased but all values were within normal range. In addition, no adverse events were observed, suggesting NKCP safety.
High dose administration(humans):
Eight healthy adults were given NKCP at 1,250mg/day for 7 consecutive days. Observation of clinical signs and laboratory tests were utilized to evaluate NKCP safety. There were no clinically significant adverse events. There were no abnormal changes in hematological or biochemistry tests.
Assays
1. Peptidase Activity (synthetic substrate method)
The sample solution is warmed at 37°C with the synthetic chromogenic substrate S-2251 (H-D-valyl-L-leucyl-L-lysine-p-nitroanilide dihydrochloride) as a substrate and the absorbance at 405nm is determined. The enzyme activity is defined as 1 unit when 1nmol of p-nitroaniline per minute is released.
*An assay is an investigative (analytic) procedure in laboratory medicine, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity (the analyte).
2. Bacillus subtilis var. natto-produced Protein
ELISA (enzyme-linked immunosorbent assay) uses rabbit-specific antibodies to the Bacillus subtilis var. natto-produced protein, responsible for the peptidase activity, to measure the amount of antigen reacting with the specific antibodies.
▶Anticoagulant effect of NKCP in human blood
Thirty micro litters of the test substance, NKCP, was added to 3mL of venous blood collected from healthy volunteers who had given informed consent, followed by inversion for mixing. The mixture was warmed at 37°C for 250 seconds and centrifuged, and the supernatant was measured for fibrin monomers (FM) indicative of thrombus formation, using a latex immunity analyzer. The concentration of FM was 160 ± 29.3μg/mL after addition of the control physiological saline and 6.0 ± 1.1μg/mL after addition of heparin sodium (0.5IU/mL). After addition of NKCP at concentrations from 0.005mg/mL to 0.5mg/mL, the concentration of FM decreased dose-dependently, but plateaued at 0.05mg/mL.
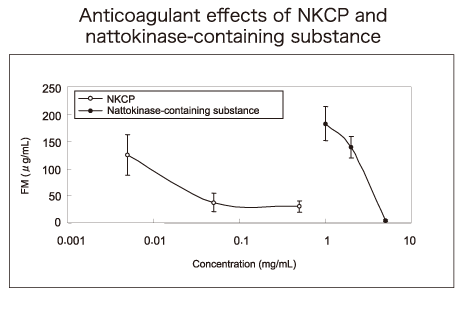
The 54th Study Meeting of Rheology 2006;
Department of Legal Medicine, Dokkyo Medical University School of Medicine
▶Anticoagulant effect of NKCP in rat model of thrombosis formation
The anticoagulant effect of NKCP was studied in a rat model of thrombus formation. In this model, platelet aggregation was caused by injuring endothelial cells of the abdominal descending aorta to induce thrombus formation. NKCP was administered into the duodenum 2 hours after producing the thrombus formation model using in situ loop method. Blood was collected from the abdominal aorta 6 hours after administration to determine activated partial thromboplastin time (APTT) and prothrombin time (PT) as indicators of endogenous and exogenous coagulations, respectively. APTT was 33.5 ± 2.4 seconds for the control group (administered physiological saline), 52.0 ± 4.5 seconds for the NKCP 100mg/kg group, and 63.3 ± 2.9 seconds for the NKCP 250mg/kg group. A significant coagulation delay was observed in the NKCP treatment groups as compared to control. PT was 16.7 ± 0.5 seconds for the control group, 20.6 ± 0.9 seconds for the NKCP 100mg/kg group, and 21.3 ± 1.7 seconds for the NKCP 250mg/kg group. As with the APTT, a significant coagulation delay was observed in the NKCP group as compared to control. These results suggest NKCP inhibits thrombus formation.
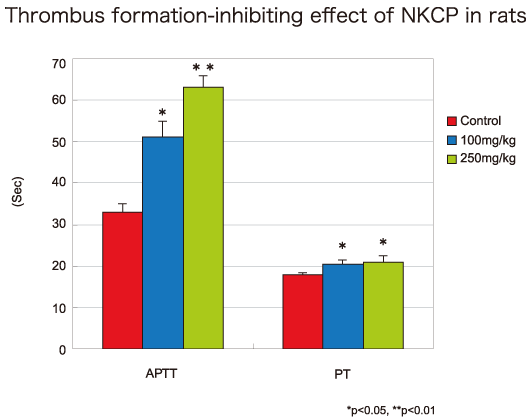

Research and Development Department, Daiwa Pharmaceutical Co., Ltd., 2001
Eight healthy male adults who had given informed consent were given a single dose of placebo or NKCP at 1,250mg, and blood was collected at various points over 240 minutes to measure changes in blood viscosity. The viscosity of blood was determined by Hitosugi et al.'s method using an oscillating viscometer.
In the NKCP group, there was a significant decrease in blood viscosity at 105 and 180 minutes as compared to baseline. There was a significant decrease in blood viscosity in the NKCP group as compared to placebo at 180 minutes.
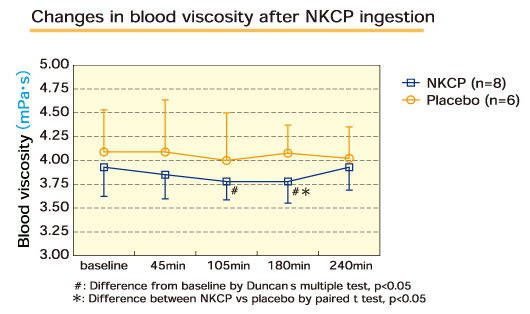
Effect of NKCP on the viscosity of human blood:
Department of Legal Medicine, Dokkyo Medical University School of Medicine.
▶Thrombolytic effect of NKCP on artificial thrombi
A small amount of NKCP was added to test tubes containing an artificial thrombus in physiological saline. The thrombus began to lyse within several minutes and was almost completely lysed at 3 hours. To lyse is to cause dissolution or destruction of cells by lysins. Lysis is the disintegration of a cell by rupture of the cell wall or membrane.
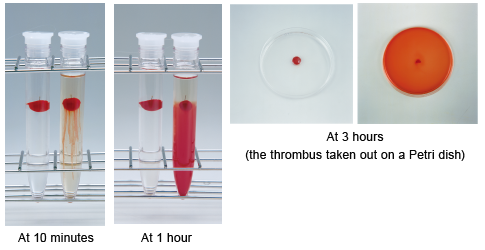
Thrombolytic effect of NKCP on artificial thrombi:
J. Pharmacol Sci 2005; 99: 247-251.
▶In vivo thrombolytic effect of oral NKCP in experimental thrombolysis model
The thrombolytic effect of NKCP on rat model was observed in a 14 week study. The NKCP was evaluated in two groups, in a mix of 0.2% and 1% in feed, as compared to a control group. Thrombolysis was evaluated using a He-Ne laser induced thrombosis model in mesenteric microvessels. The size of the artificially produced thrombus was measured from the time of formation to evaluate the thrombolytic effect of NKCP.
Thrombolytic activity clearly increased dose-dependently in the NKCP treatment groups compared with the control group. There was an 82% decrease in thrombus volume in the control group, as compared to 67% decrease in the 0.2% NKCP group, and a 51% decrease (statistically significant) in the 1% NKCP group. The extent of thrombolysis in the 1% group was equivalent to that seen in animals treated with a bolus intravenous infusion of 0.2mg/kg of tissue plasminogen activator (t-PA). Based on the body weight and feed intake of rats used in the study, the dose of NKCP was calculated to be about 160mg/kg/day for the 0.2% NKCP feed and 800mg/kg/day for the 1% NKCP feed.
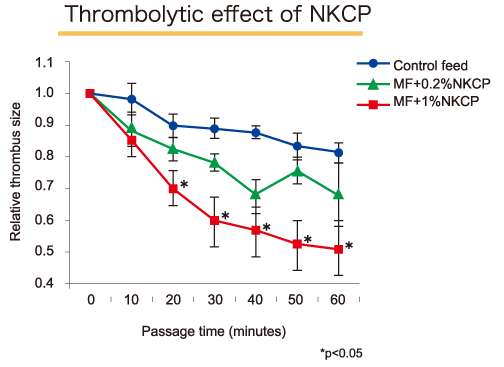
In vivo thrombolytic effect of oral NKCP in experimental thrombolysis model:
Pathophysiol Haemost Thromb 2003; 33: 138-143.
▶Identification of proteases derived from Bacillus subtilis var. natto related to thrombolysis
The thrombolytic effect of NKCP is attributable to a proteolytic enzyme named bacillopeptidase F, produced by Bacillus subtilis var. natto. Bacillopeptidase F is shown to be one of five proteases produced and secreted by Bacillus subtilis var. natto (Table 1).
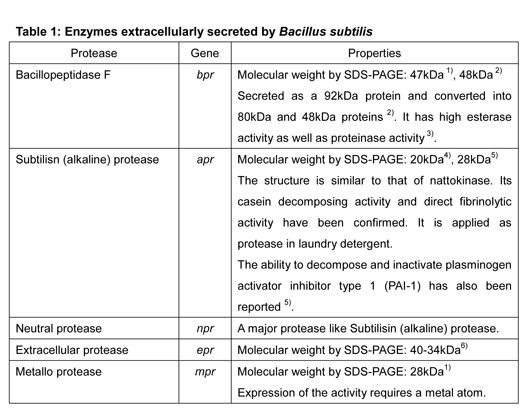
Identification of proteases derived from Bacillus subtilis natto related to thrombolysis:
1)Journal of Bacteriology 1990; 172: 1019-1023
2)The Journal of Biological Chemistry 1990; 265: 6845-6850
3)Journal of Bacteriology 1990; 172: 1470-1477
4)Experientia 1987; 43: 1110-1111
5)The Journal of Biological Chemistry 2001; 276: 24690-24696
6)Mol Gen Genet 1990 May; 221(3): 486-490
▶Effect of Bacillus subtilis var. natto-derived protein on the human blood coagulation/fibrinolysis system
A total of 23 adults, including patients with metabolic diseases related to thrombosis, were given 250mg NKCP for two consecutive months. Coagulation/fibrinolysis parameters and symptoms were evaluated 1 and 2 months after starting treatment.
Euglobulin lysis time (ELT) significantly decreased from the baseline value at 1 and 2 months, and t-PA significantly increased at 2 months, showing the accelerated fibrinolysis system. However, the measured ELT and t-PA values were within the normal ranges. Fibrinogen degradation products (FDP) significantly decreased 1 month after starting ingestion but returned to the baseline value at 2 months.
Symptoms of shoulder stiffness significantly improved from baseline at 1 and 2 months.
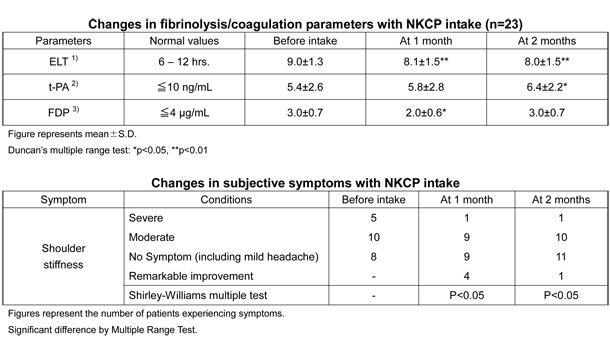
Effect of Bacillus subtilis natto-derived protein on the human blood coagulation/fibrinolysis system:
Journal of the Japanese Society of Biorheology 2004; 18 (1).

 Heart disease and cerebrovascular
Heart disease and cerebrovascular 
 Daiwa Pharmaceutical Co. is a new breed of pharmaceutical company focused entirely on the development of natural food solutions. Already renowned for producing what is now the leading serious immune system supplement, BioBran MGN-3, a food extract made entirely from rice bran, Daiwa Pharmaceutical have now developed the highest quality and most active Natto extract (sometimes called nattokinase, although this is just one of its components) — NKCP. What sets Daiwa Pharmaceutical apart from many other developers of innovative foods and food supplements is its dedication to high quality research and production. Daiwa is not afraid to developing natural (and therefore not patentable) products, something which most pharmaceutical companies would be reluctant to do.
Daiwa Pharmaceutical Co. is a new breed of pharmaceutical company focused entirely on the development of natural food solutions. Already renowned for producing what is now the leading serious immune system supplement, BioBran MGN-3, a food extract made entirely from rice bran, Daiwa Pharmaceutical have now developed the highest quality and most active Natto extract (sometimes called nattokinase, although this is just one of its components) — NKCP. What sets Daiwa Pharmaceutical apart from many other developers of innovative foods and food supplements is its dedication to high quality research and production. Daiwa is not afraid to developing natural (and therefore not patentable) products, something which most pharmaceutical companies would be reluctant to do.









