Learn more brain 🧠 network:长期书法书写训练对大脑网络效率有积极影响
Long-term Chinese calligraphic handwriting training has a positive effect on brain network efficiency
Abstract
As a visual art form, Chinese calligraphic handwriting (CCH) has been found to correlate with certain brain activity and to induce functional connectivity reorganization of the brain. This study investigated the effect of long-term CCH training on brain functional plasticity as assessed with network measures. With the resting-state fMRI data from 31 participants with at least five years of CCH training and 40 controls, we constructed brain functional networks, examined group differences at both the whole brain and modular levels, and correlated the topological characteristics with calligraphy skills. We found that, compared to the control group, the CCH group showed shorter characteristic path lengths and higher local efficiency in certain brain areas in the frontal and parietal cortices, limbic system, basal ganglia, and thalamus. Moreover, these network measures in the cingulate cortex, caudate nucleus, and thalamus were associated with CCH performance (i.e., copying and creating skills). These results suggest that long-term CCH training has a positive effect on the topological characteristics of brain networks.
作为一种视觉艺术形式,中国书法手写(CCH)已被发现与某些大脑活动相关并诱导大脑功能连接重组。本研究探讨了长期 CCH 训练对大脑功能可塑性的影响,并通过网络测量进行评估。利用来自 31 名至少接受过五年 CCH 训练的参与者和 40 名对照者的静息态 fMRI 数据,我们构建了大脑功能网络,检查了全脑和模块水平的组间差异,并将拓扑特征与书法技能相关联。我们发现,与对照组相比,CCH 组在额叶和顶叶皮质、边缘系统、基底神经节和丘脑的某些大脑区域表现出更短的特征路径长度和更高的局部效率。此外,扣带皮层、尾状核和丘脑中的这些网络测量与 CCH 表现(即复制和创作技能)相关。 这些结果表明长期 CCH 训练对大脑网络拓扑特征有积极的影响。
1. Introduction
1. 简介
Chinese calligraphic handwriting (CCH) is a 3000-year-old art form. To master CCH skills requires years of intensive practice that involves sensory perception, motor skills, as well as multiple cognitive and emotional elements [1, 2]. Following previous research that found both structural and functional brain plasticity in response to many types of intensive training such as musical training [3, 4], driving [5], and juggling [6, 7], we have examined brain plasticity related to CCH training. Our previous two studies found that CCH training strengthened the RSFC of brain areas involved in updating and inhibition [8] and decreased the volume of the posterior cingulate cortex (PCC) [9].
中国书法(CCH)是一种有3000年历史的艺术形式。要掌握CCH技能需要多年的密集练习,其中涉及感觉知觉、运动技能以及多种认知和情感元素[1,2]。先前的研究发现,大脑在结构和功能上都表现出可塑性,可以应对多种类型的强化训练,例如音乐训练[3,4]、驾驶[5]和杂耍[6,7],我们研究了与CCH训练相关的大脑可塑性。我们之前的两项研究发现,CCH训练增强了与更新和抑制有关的大脑区域的RSFC [8],并减少了后扣带皮层(PCC)的体积[9]。
In addition to the traditional univariate neuroimaging methods such as voxel-based morphometry (VBM) and resting-state functional connectivity (RSFC) used in the studies mentioned above, researchers have recently paid attention to brain connectivity networks or modular organization. Brain network analysis can mathematically describe various topological parameters of the brain’s organization in terms of graphs or networks, including the small-worldness, modularity, and regional network parameters [10, 11]. Studies have proved that functionally connected resting-state brain networks are associated with the anatomical connectivity of the brain [12, 13].
除了上述研究中使用的传统单变量神经成像方法,例如基于体素的形态测量法 (VBM) 和静息态功能连接法 (RSFC) 外,近年来,研究人员开始关注脑连接网络或模块组织。脑网络分析可以用图形或网络的形式,以数学形式描述脑组织的各种拓扑参数,包括小世界性、模块性和区域网络参数 [10, 11]。研究证明,功能连接的静息态脑网络与脑的解剖连接相关 [12, 13]。
Given our previous findings of CCH training’s effects on the RSFC of certain brain areas [8], we hypothesized long-term CCH practicing would have an effect on the topological parameters of the resting-state brain network, including the frontal and parietal cortices, basal ganglia, and PCC. We explored the long-term CCH training’s effect on the topological characteristics of the whole brain and four specific modules. These modules were selected because of their relevance to visual processing (Module I), sensorimotor functions (Module II), and DMN (Module III), all of which are involved in CCH. More details of the brain areas included in each module are shown Fig 1 and S1 Table. Finally, within the CCH group, we further investigated the relationship between global and local network measures and calligraphy skills.
鉴于我们之前关于 CCH 训练对某些脑区 RSFC 的影响的研究结果 [8],我们假设长期 CCH 练习会对静息态脑网络的拓扑参数产生影响,包括额叶和顶叶皮质、基底神经节和 PCC。我们探讨了长期 CCH 训练对整个大脑和四个特定模块的拓扑特征的影响。之所以选择这四个模块,是因为它们与视觉处理(模块 I)、感觉运动功能(模块 II)和默认模式网络(模块 III)相关,而这些功能都与 CCH 有关。每个模块所包含的脑区的更多细节显示在图 1 和 S1 表中。最后,在 CCH 组中,我们进一步研究了全局和局部网络测量值与书法技能之间的关系。
Fig 1. Visualization of the four modules selected for network efficiency analyses.
图 1. 用于网络效率分析的四个模块的可视化。
Fig 1:Open in a new tab ( click here to view )
图 1:在新标签页中打开(点击此处查看)
Modules I, II, and III mean the sets of brain areas involved in visual processing, sensorimotor functions, and the DMN, respectively. L: left hemisphere; R: right hemisphere.
模块 I、II 和 III 分别表示与视觉处理、感觉运动功能和默认模式网络 (DMN) 相关的脑区集合。L:左半球;R:右半球。
2. Materials and methods
2.材料和方法
2.1. Participants
2.1. 参与者
Participants were recruited from Beijing Normal University, Beijing, China. The CCH group included 32 students who majored in calligraphy and had at least five years of formal training in CCH and the control group included 44 students who had no more than a few months of basic CCH skill training. All subjects were right-handed native Chinese speakers. Participants’ IQ was measured with Raven’s Advanced Progressive Matrices (APM) (for details, see Chen et al., 2017) [8]. Each participant signed an informed consent form after a full explanation of the study procedure. This study was approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neuroscience and Learning at Beijing Normal University, China. Subjects were compensated for their time.
参与者来自北京师范大学。CCH组包括32名书法专业学生,他们至少接受过五年的CCH正规训练;对照组包括44名学生,他们接受过不超过几个月的基本CCH技能训练。所有受试者均为右利手汉语母语人士。参与者的智商采用瑞文高级渐进矩阵测验(APM)进行测量(详情请参阅陈等人,2017)[8]。在充分解释研究程序后,每位参与者都签署了知情同意书。本研究经北京师范大学认知神经科学与学习国家重点实验室机构审查委员会批准。受试者将获得时间补偿。
2.2 Calligraphy skills
2.2 书法技巧
Assessment of participants’ calligraphy skills was based on their performance on two tasks: copying a famous calligraphy work and creating a new calligraphy work.
对参与者书法技能的评估基于他们在两项任务中的表现:临摹著名书法作品和创作新书法作品。
All participants of the CCH group were asked to copy a part of a calligraphy masterpiece titled “the Northern Wei sculpture’ and to create a calligraphy work (in any style) using an existing quatrain written by Qi Gong, a celebrated cultural figure in China.
CCH 组的所有参与者都被要求临摹书法杰作《北魏雕塑》的一部分,并使用中国著名文化人物启功的一首现有绝句创作书法作品(风格不限)。
Participants’ works (with identity information removed) were evaluated by seven calligraphy teachers from the Department of Calligraphy of the School of Arts and Media, Beijing Normal University. They used a 10-point scoring system (from 1 to 10 with interval of 0.5). The final score for each piece of work was the mean of the five non-extreme scores after eliminating two extreme scores (the highest and the lowest) (see the Results section for interjudge reliability).
参与者的作品(已删除身份信息)由北京师范大学艺术与传媒学院书法系7位书法教师进行评分。评分采用10分制(1-10分,每分0.5)。每件作品的最终得分为剔除两个极端分数(最高分和最低分)后,取5个非极端分数的平均值(评委间信度见结果部分)。
2.3 Brain imaging data collection and preprocessing
2.3 脑成像数据采集与预处理
2.3.1 fMRI data acquisition
2.3.1 fMRI数据采集
All scanning was performed using a SIEMENS TRIO 3-Tesla scanner in the Brain Imaging Center of Beijing Normal University. Participants were told neither to have heavy physical activities nor have stimulating drinks the day before the scanning. Each participant underwent a 3D anatomic session and an eight-minute resting-state fMRI (RS-fMRI) scanning session. The 3D T1-weighted magnetization- prepared rapid gradient echo (MPRAGE) image was acquired with the following parameters: 144 sagital slices, slice thickness/gap = 1.3/0.65 mm, TR = 2530 ms, TE = 3.39 ms, inversion time (Ti) = 1100 ms, flip angle = 7°, FOV = 256×256 mm2, matrix size = 256×192. During the RS-fMRI session, the participants were instructed to keep their eyes closed, be as still as possible, and not to think about anything in particular. Images were obtained with the following parameters: 33 axial slices, thickness/gap = 3.5/0.7 mm, matrix size = 64×64, repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 200×200 mm2.
所有扫描均在北京师范大学脑影像中心使用西门子TRIO 3特斯拉扫描仪进行。扫描前一天告知受试者不要进行剧烈运动或饮用刺激性饮料。每位受试者均接受3D解剖和8分钟的静息态fMRI(RS-fMRI)扫描。3D T1加权磁化制备的快速梯度回波(MPRAGE)图像采用以下参数采集:144个矢状面切片,切片厚度/间隙=1.3/0.65 mm,TR=2530 ms,TE=3.39 ms,反转时间(Ti)=1100 ms,翻转角=7°,FOV=256×256 mm2,矩阵大小=256×192。 在RS-fMRI检查过程中,受试者被要求闭眼,尽可能保持静止,并且不要思考任何特定的事情。图像采集参数如下:33个轴向切片,厚度/间隙=3.5/0.7 mm,矩阵大小=64×64,重复时间(TR) = 2000 ms,回波时间(TE) = 30 ms,翻转角= 90°,视场(FOV) = 200×200 mm²。
2.3.2 Image preprocessing and analysis
2.3.2 图像预处理与分析
Resting-State fMRI data were preprocessed using DPARSE (DPARSF, http://rfmri.org/DPARSF) [14]. Briefly, after discarding the first 10 volumes, the following steps were performed: correction, coregistration, segmentation, normalization, smoothing, linear detrending, regressing nuisance signals, and filtering. For more detail, see Chen et al. (2017).
使用 DPARSE (DPARSF,http://rfmri.org/DPARSF) [14] 对静息态 fMRI 数据进行预处理。简而言之,在丢弃前 10 个体素后,执行以下步骤:校正、配准、分割、归一化、平滑、线性去趋势、回归干扰信号和滤波。更多详细信息,请参阅 Chen 等人 (2017) 的文章。
In order to construct the connectivity network, the preprocessed RS-fMRI images data were overlapped with ALL templates (90 cortical and 26 cerebellar anatomical areas), and then the mean time series of all the 116 nodes were calculated in REST [15]. Pearson correlation was performed to obtain ‘r-value matrix’ and then Fisher’s r-to-z transformation was employed to obtain ‘z-score matrix’.
为了构建连接网络,将预处理的RS-fMRI图像数据与ALL模板(90个皮层和26个小脑解剖区域)叠加,然后在REST[15]中计算所有116个节点的平均时间序列。进行皮尔逊相关分析以获得“r值矩阵”,然后采用Fisher的r-to-z变换以获得“z得分矩阵”。
2.4 Statistics
2.4 统计
After obtaining the correlation matrix, we used GraphVar (http://rfmri.org/GraphVar), a GUI-based toolbox for graph theoretical methods [16], to calculate the global and local clustering coefficient (Cp), global and local characteristic path length (Lp), global efficiency (Eglob), and local efficiency (Eloc) of the whole gray matter brain (90 ALL brain areas) and four modules with a range of network cost (0.1~0.5). We also generated 100 binary random networks per subject per threshold (null-model networks) to test the difference between network measures and the random networks, and also tested the small-worldness trait of human brain resting-state network. With age, gender and IQ as covariates, we then performed group analyses with 1000 permutations (corrected p <.05), which is a non-parametric testing method to detect the statistical significance of the group differences in network topological characteristics [16, 17]. The analyses were conducted both for the whole brain and the four modules.
得到相关矩阵后,我们使用基于图形用户界面的图论方法工具箱 [16] GraphVar (http://rfmri.org/GraphVar) 计算了整个灰质脑(90 个脑区)和四个模块的全局和局部聚类系数 (Cp)、全局和局部特征路径长度 (Lp)、全局效率 (Eglob) 和局部效率 (Eloc),网络成本范围为 (0.1~0.5)。我们还为每个受试者每个阈值生成了 100 个二元随机网络(零模型网络)来测试网络测度与随机网络之间的差异,并测试了人脑静息态网络的小世界特性。然后,我们以年龄、性别和智商为协变量,进行了 1000 次排列的组分析(校正 p <.05),这是一种非参数检验方法,用于检测网络拓扑特征组间差异的统计学意义 [16, 17]。 分析针对整个大脑和四个模块进行。
Within the CCH group, we also correlated calligraphy skills (copying scores and creating scores) with topological characteristics (global and local Cp, Lp, Eglob and Eloc).
在 CCH 组中,我们还将书法技能(抄写乐谱和创作乐谱)与拓扑特征(全局和局部 Cp、Lp、Eglob 和 Eloc)关联起来。
3. Results
3.结果
3.1 Reliability analysis of the calligraphy skills
3.1 书法技能的信度分析
We used Kendall’s coefficient of concordance to assess the agreement among the seven judges of the calligraphy skills. The Kendall’s W value was 0.719 (p< 0.001) for copying skills and 0.800 (p< 0.001) for creating skills. Because four of the seven judges were the participants’ teachers, we correlated the average ratings of the four calligraphy teachers of the participants and those of the three judges who had not taught these participants. Results showed very high correlations between them for the two measures of quality of CCH: the coping scores (r =.882, p <.01) and creating scores (r =.863, p <.01). These results suggest that even if the teachers might have been able to infer the identities of the participants from the anonymized writings, they were making similar judgments as the blind judges.
我们使用 Kendall 的一致性系数来评估七位书法技能评委之间的一致性。抄写技能的 Kendall W 值为 0.719(p< 0.001),创作技能的 Kendall W 值为 0.800(p< 0.001)。因为七位评委中有四位是参与者的老师,所以我们将参与者的四位书法老师的平均评分与三位没有教过这些参与者的评委的平均评分关联起来。结果显示,对于 CCH 的两个质量指标,他们之间存在非常高的相关性:应对分数(r = .882,p < .01)和创作分数(r = .863,p < .01)。这些结果表明,即使老师可能能够从匿名书写中推断出参与者的身份,他们做出的判断与盲评类似。
3.2 Demographic data
3.2 人口统计数据
There was no group difference between the CCH and control groups in terms of gender, age (CCH group: 21.23±2.11; control group: 21.61±2.54), years of education (CCH group: 14.34±2.04; control group: 14.59±1.85), and IQ (CCH group: 26.47±3.68; control group: 27.58±3.74). The CCH practitioners had an average of 10 years of experience and started practicing at around 9 years of age (for more details, see Chen et. al, (2017).
中医组与对照组在性别、年龄(中医组:21.23±2.11,对照组:21.61±2.54)、受教育年限(中医组:14.34±2.04,对照组:14.59±1.85)和智商(中医组:26.47±3.68,对照组:27.58±3.74)方面均无组间差异。中医执业者平均从业年限为10年,平均从9岁左右开始行医(详见陈等,2017)。
Copying score was correlated negatively with the onset age of CCH practice (r = -0.627, p<0.001) and positively with the number of years of practice (r = 0.390, p = 0.021). Creating skills were significantly correlated with only the onset age of practice (r = -0.347, p = 0.041) (Table 1).
抄写技能得分与开始练习CCH的年龄呈负相关(r = -0.627,p<0.001),与练习年限呈正相关(r = 0.390,p = 0.021)。创造技能得分仅与开始练习的年龄显著相关(r = -0.347,p = 0.041)(表1)。
Table 1. The relationship between the time factors of CCH practice and calligraphy skills.
表1. 书法练习的时间因素与书法技能的关系。
Copying score 抄袭乐谱
Creating score 创建乐谱
r p r p
Onset age of practice 开始练习的年龄 -0.627 0.000 -0.347 0.041
Yeas of practice 多年实践 0.390 0.021
0.164 0.346
Open in a new tab , click here
在新标签页中打开,点击此处
3.3 Topological characteristics of the RSFC networks
3.3 RSFC网络的拓扑特征
3.3.1 Whole brain network
3.3.1 全脑网络
Six topological properties, the global Cp and Lp, local Cp and Lp, Eglob and Eloc, were used to characterize the global and local topological properties of the brain network. The two groups did not differ in small-world characteristics of brain organization (S1 Fig). They also did not differ in global Cp, Lp and Eglob. For the local parameters, we found that compared to the control group, the CCH group showed larger local Cp in right middle frontal gyrus, left inferior triangle frontal gyrus, left anterior and median cingulum, right hippocampus gyrus, right inferior frontal gyrus, right anterior cingulum, right calcarine fissure, and right fusiform gyrus (Table 2). The CCH group also showed shorter local Lp in right anteriror cingulum, left supramarginal gyrus, left caudate nucleus, and bilateral thalamus, but longer Lp in right hippocampus and caudate nucleus (Table 3). Finally, the CCH group showed higher Eloc in right PCC, right superior and inferior parietal gyrus, caudate nucleus, and thalamus (Table 4).
用全局 Cp 和 Lp、局部 Cp 和 Lp、Eglob 和 Eloc 六种拓扑特性来表征脑网络的全局和局部拓扑特性。两组在脑组织的小世界特征上没有差异(S1 图),在全局 Cp、Lp 和 Eglob 上也没有差异。对于局部参数,我们发现与对照组相比,CCH 组在右侧中额回、左侧下三角额回、左侧前中扣带回、右侧海马回、右侧下额回、右侧前扣带回、右侧距状裂和右侧梭状回表现出较大的局部 Cp(表 2)。 CCH 组还表现出右侧前扣带回、左侧缘上回、左侧尾状核和双侧丘脑的局部 Lp 缩短,但右侧海马和尾状核的 Lp 延长(表 3)。最后,CCH 组表现出右侧 PCC、右侧顶上回和顶下回、尾状核和丘脑的 Eloc 升高(表 4)。
Table 2. Brain regions with significant group differences in characteristic path length between the CCH and control groups (corrected p <.05).
表 2. CCH 组和对照组之间特征路径长度存在显著组间差异的大脑区域(校正 p <.05)。
Series AAL Template Mean t value Mean p value
32 Right anterior cingulate cortex -0.387 0.039
38 Right hippocampus 0.665 0.037
63 Left supramarginal gyrus -1.990 0.031
71 Left caudate nucleus -1.815 0.013
72 Right caudate nucleus 0.300 0.010
77 Left thalamus -0.706 0.025
78 Right thalamus -1.615 0.024
Open in a new tab, here
在新标签页中打开,点击此处
Note: Positive t values mean that the CCH group had longer paths, whereas negative t values mean that the control group had longer paths.
注意:正 t 值表示 CCH 组的路径较长,而负 t 值表示对照组的路径较长。
Table 3. Brain regions with significant group differences in local clustering coefficient between the CCH and control groups (corrected p <.05).
Series AAL Temple Mean t value Mean p value
8 Right middle frontal gyrus 0.267 0.033
14 Right inferior frontal gyrus -0.778 0.043
15 Left inferior triangel frontal gyrus 1.166 0.045
31 Left anterior cingulate cortex 1.372 0.035
32 Right anterior cingulate cortex -1.086 0.026
33 Left median cingulate cortex 0.057 0.040
34 Right median cingulate cortex 1.612 0.011
38 Right hippocampus gyrus 2.028 0.034
44 Right calcarine fissure -0.368 0.039
56 Right fusiform gyrus -0.679 0.007
.
Note: Positive t values mean that the CCH group had larger coefficients, whereas negative t values mean that the control group had larger coefficients.
Table 4. Brain regions with significant differences in local efficiency between the CCH and control groups (corrected p <.05).
Series AAL Template Mean t value Mean p value
36 Right post cingulate cortex 0.292 0.019
60 Right superior parietal gyrus 0.317 0.034
62 Right inferior parietal gyrus 0.184 0.042
63 Left supramarginal gyrus -0.465 0.023
72 Right caudate nucleus 0.683 0.027
78 Right thalamus 0.978 0.018
Open in a new tab
Note: Positive t values mean that the CCH group had higher local efficiency, whereas negative t values mean that the control group had higher local efficiency.
3.3.2 Group difference in modular network efficiency
3.3.2 模块网络效率的组间差异.
We chose four modular networks that are most relevant to CCH training for the network efficiency analysis. Module I (visual processing) showed that the CCH group had high lower Cp in right calcarine fissure and higher Cp in left MOG than did the control group (corrected alpha level of 0.05 with 1000 permutations, at the threshold of 0.19–0.45). The CCH group also showed longer Lp in right inferior occipital gyrus and shorter Lp in left fusiform. The CCH group showed higher Eloc in bilateral middle occipital gyrus (Fig 2). Finally, no group difference was found in terms of Eglobal in Module I.
我们选择了与 CCH 训练最相关的四个模块网络进行网络效率分析。模块 I(视觉处理)显示,CCH 组右侧距状裂的 Cp 较低,左侧 MOG 的 Cp 较高,均高于对照组(校正后的 alpha 水平为 0.05,1000 次排列,阈值为 0.19–0.45)。CCH 组右侧枕下回的 Lp 较长,左侧梭状回的 Lp 较短。CCH 组双侧枕中回的 Eloc 较高(图 2)。最后,在模块 I 中,Eglobal 方面未发现组间差异。
Fig 2. Bilateral middle occipital region showed higher Eloc in the CCH group than in the control group (red) in Module I, with 1000 permutations (p <.05).
Fig 2 : Open in a new tab ( here )
图 2:在新标签页中打开(此处)
Note: 1. Calcarine_L; 2. Calcarine_R; 3. Cuneus_L; 4. Cuneus_R; 5. Lingual_L; 6. Lingual_R; 7. Occipital_Sup_L; 8. Occipital_Sup_R; 9. Occipital_Mid_L; 10. Occipital_Mid_R; 11. Occipital_Inf_L; 12. Occipital_Inf_R; 13. Fusiform_L; 14. Fusiform_R.
注:1. Calcarine_L; 2. Calcarine_R; 3. 楔形肌_L; 4. Cuneus_R; 5. 语言_L; 6. 语言_R; 7. 枕骨_Sup_L; 8. 枕骨_Sup_R; 9. 枕骨_中_L; 10. 枕骨_中_R; 11. 枕骨_Inf_L; 12. 枕骨_信息_R; 13. Fusiform_L; 14.Fusiform_R。
Contrary to our expectations, Module II (the sensorimotor areas) showed no significant group differences. For Module III (the DMN), the CCH group showed higher Cp in left superior and medial frontal gyrus and lower Cp in bilateral precuneus than did the control group. The CCH group also showed significantly shorter Lp when the network threshold was 0.15, 0.16, 0.19 and 0.20. At the local level, the CCH group showed significantly shorter Lp than the control group in left anterior cingulum and bilateral precuneus, but higher Lp in right caudate nucleus. In contrast, the CCH group showed significantly higher Eloc in bilateral precuneus, and lower Eloc in bilateral caudate nucleus (Fig 3). The CCH group tended to have higher Eglobal than the control group, which reached significance at two network thresholds (0.16 and 0.20) (S2 Fig).
与我们的预期相反,模块 II(感觉运动区)没有显示出明显的组间差异。对于模块 III(DMN),与对照组相比,CCH 组的左侧上额叶和内侧额叶的 Cp 较高,而双侧楔前叶的 Cp 较低。当网络阈值为 0.15、0.16、0.19 和 0.20 时,CCH 组的 Lp 也显著较短。在局部层面,CCH 组的左侧前扣带回和双侧楔前叶的 Lp 显著短于对照组,但右侧尾状核的 Lp 较高。相反,CCH 组的双侧楔前叶的 Eloc 显著较高,双侧尾状核的 Eloc 较低(图 3)。CCH 组的 Eglobal 倾向于高于对照组,这在两个网络阈值(0.16 和 0.20)处均达到显著性(S2 图)。
Fig 3. The CCH group showed higher Eloc in bilateral precuneus (red) and lower in bilateral caudate nucleus (green) than the control group in Module III.
图 3. 在模块 III 中,CCH 组双侧楔前叶(红色)的 Eloc 高于对照组,双侧尾状核(绿色)的 Eloc 低于对照组。
Fig 3 : Open in a new tab (here)
图 3:在新标签页中打开(此处)
Note: 1. Frontal_Sup_L (dorsolateral); 2. Frontal_Sup_R (dorsolateral); 3. Frontal_Mid_L; 4. Frontal_Mid_R; 5. Frontal_Mid_Orb_L; 6. Frontal_Sup_Medial_L; 7. Frontal_Sup_Medial_R; 8. Cingulum_Ant_L (Anterior cingulate and paracingulate gyri); 9. Angular_L; 10. Angular_R; 11. Precuneus_L; 12. Precuneus_R; 13. Caudate_L; 14. Caudate_R; 15. Thalamus_L; 16.Thalamus_R.
3.4 Correlation analyses between brain network parameters and calligraphy skills
3.4 脑网络参数与书法技能的相关性分析
The above results showed group differences in topological characteristics (shorter Lp and higher Eloc for the CCH group than the control group). To extend those results to individual differences within the CCH group, we conducted correlation analyses between brain network parameters and calligraphy skills. The correlational analyses generally confirmed those associations within the CCH group. Specifically, within thresholds 0.13~0.21, copying score was negatively correlated with characteristic path length at the global level. At the local level, the Lp of certain brain regions (i.e., bilateral olfactory cortex, amygdala, caudate nucleus; right rectus, thalamus and middle temporal pole) all showed negative relationship with copying score, whereas the Lp of left thalamus was negatively correlated with creating score and the Lp of left inferior occipital gyrus and bilateral caudate nucleus was positively correlated with creating score.
上述结果显示拓扑特征存在组间差异(CCH组的Lp比对照组短而Eloc比对照组高)。为了将这些结果推广到CCH组内的个体差异,我们进行了脑网络参数与书法技能的相关性分析。相关性分析普遍证实了CCH组内的这种关联。具体而言,在阈值0.13~0.21内,在整体层面上,抄写分数与特征路径长度呈负相关。在局部层面上,某些脑区(即双侧嗅皮质、杏仁核、尾状核;右侧直肌、丘脑和颞中极)的Lp均与抄写分数呈负相关,而左侧丘脑的Lp与创作分数呈负相关,左侧枕下回和双侧尾状核的 Lp与创作分数呈正相关。
Cp in the right supplementary motor areas, right superior occipital gyrus, and left inferior occipital gyrus was negatively correlated with copying scores, but Cp in right gyrus rectus and amygdala was positively correlated with copying score. Cp in the right calcarine fissure, cuneus, superior and middle occipital gyrus, middle temporal gyrus, and left inferior occipital gyrus was negatively correlated with creating score, and that of right inferior frontal gyrus was positively correlated with creating score.
右侧辅助运动区、右侧枕上回、左侧枕下回的Cp与复制得分呈负相关,右侧直回、杏仁核的Cp与复制得分呈正相关;右侧距状裂、楔叶、枕上回、枕中回、颞中回、左侧枕下回的Cp与创造得分呈负相关,右侧额下回的Cp与创造得分呈正相关。
The Eloc of bilateral superior medial frontal gyrus and PCC, right hippocampus and lenticular nucleus (pallidum), and left temporal pole (middle temporal gyrus) showed positive relationship with copying score. The Eloc of left supplementary motor area, insula, bilateral paracentral lobule, caudate nucleus and thalamus, and right lenticular nucleus (pallidum) was positively correlated with creating score, but Eloc of left inferior occipital gyrus was negatively correlated with creating score (Table 5).
双侧额上回及PCC、右侧海马及豆状核(苍白球)、左侧颞极(颞中回)的Eloc与复制评分呈正相关,左侧辅助运动区、岛叶、双侧中央旁小叶、尾状核及丘脑、右侧豆状核(苍白球)的Eloc与创造评分呈正相关,左侧枕下回的Eloc与创造评分呈负相关(表5)。
Table 5. The positive (+) and negative (-) correlation brain regions with r values between local topological characteristics (i. e., Lp, Cp, Eloc) and calligraphy skills.
表 5. 局部拓扑特征(即 Lp、Cp、Eloc)与书法技能之间的正(+)和负(-)相关脑区及其 r 值。
Copying score Creating score
+ - + -
Lp N left olfactory cortex (-0.374) left inferior occipital gyrus (0.567) left thalamus (-0.249)
right olfactory cortex (-0.362) left caudata nucelus (0.170)
left amygdala (-0.453) right caudata nucelus (0.150)
right amygdala (-0.558)
left caudate nucelus (-0.317)
right caudate nucelus (-0.268)
right rectus (-0.439)
right thalamus (-0.346)
right middle temporal pole (-0.623)
Cp right gyrus rectus (0.378) right supplementary motor areas (-0.566) right inferior frontal gyrus (0.524) right calcarine fissure (-0.356)
right amygdala (0.438) right superior occipital gyrus (-0.482) right cuneus (-0.359)
left inferior occipital gyrus (-0.456) right superior occipital gyrus (-0.395)
right middle occipital gyrus (-0.357)
right middle temporal gyrus (-0.345)
left inferior occipital gyrus (-0.386)
Eloc left superior medial frontal gyrus (0.604) right paracentral lobule (-0.355) left supplementary motor area (0.422) left inferior occipital gyrus (-0.440)
right superior medial frontal gyrus (0.671) right caudate nucelus (-0.118) left insula (0.439)
left post cingulum cortex (0.567) left paracentral lobule (0.366)
right post cingulum cortex (0.657) right paracentral lobule (0.257)
right hippocampus (0.442) left caudata nucelus (0.156)
right lenticular nucleus (pallidum) (0.534) right caudata nucelus (0.182)
left temporal pole (middle temporal gyrus) (0.419) left thalamus (0.164)
right thalamus (0.188)
right lenticular nucleus (pallidum) (0.387)
Open in a new tab
Note: + positive correlation (P < 0.05),
− negative correlation (P < 0.05),
N no significant correlation brain regions (P > 0.05).
Taken together, we found that the cingulate cortex, caudate nucleus and thalamus were the core brain areas that showed both group differences between the CCH and control groups and significant correlations with calligraphy skills within the CCH group (Table 6).
综合起来,我们发现扣带皮层、尾状核和丘脑是CCH组与对照组之间表现出群体差异以及与CCH组内书法技能具有显著相关性的核心脑区(表6)。
Table 6. Group differences and neural correlates (within the DMN) of CCH skills.
表 6. CCH 技能的群体差异和神经相关性(在 DMN 内)。
Brain areas Group difference Correlation
Coping score Creating score
Lp Eloc Lp Eloc Lp Eloc
Left anterior cingulate cortex
Right anterior cingulate cortex -
Left post cingulate cortex +
Right post cingulate cortex + +
Left caudate nucleus - - + +
Right caudate nucleus + + - - + +
Left thalamus - - +
Right thalamus - + - +
Open in a new tab
+ means the CCH group had longer Lp/higher Eloc than the control group or positive correlation.
− means the CCH group had shorter Lp/lower Eloc than the control group or negative correlation (P < 0.05).
4. Discussion
4.讨论
The current study explored the effect the long-term experience with CCH on brain network efficiency assessed with parameters based on graph theory. We found that compared to the controls, individuals with long-term CCH training showed advantages in topological characteristics (i.e., Lp, Cp and Eloc) in certain brain areas based on both whole brain and modular analyses. Moreover, within the CCH group, calligraphy skills were associated with brain network efficiency parameters, especially Lp and Eloc.
本研究探讨了长期接受CCH训练对大脑网络效率的影响,并以基于图论的参数进行评估。我们发现,与对照组相比,接受长期CCH训练的个体在某些脑区的拓扑特征(即Lp、Cp 和 Eloc)上表现出优势(基于全脑和模块分析)。此外,在CCH组中,书法技能与大脑网络效率参数(尤其是 Lp 和 Eloc)相关。
Seven brain regions showed significant group differences in Lp (Table 3), with the CCH group having shorter Lp than the control group for five of the seven regions. It appears that CCH training increased the information transfer speed (as indexed by Lp from one brain area to another). Consistent with the group differences, copying score had a significant negative relationship with Lp within CCH participants. Similar to the results with Lp, the CCH group had higher Eloc than the control group in all brain areas in the right hemisphere and Eloc in many of these brain regions was positively correlated with calligraphy skills (including both copying and creating scores) of CCH participants. Those results demonstrated that CCH training improved the partial information network’s topological structure of certain brain areas.
七个脑区的Lp表现出明显的组间差异(表3),CCH组在七个脑区中的五个脑区的Lp比对照组短。CCH训练似乎提高了信息传递速度(以Lp为指标从一个脑区到另一个脑区的传递速度)。与组间差异一致,CCH组参与者的抄写成绩与Lp呈显著负相关。与Lp的结果类似,CCH组在右半球所有脑区的Eloc都高于对照组,并且这些脑区的Eloc与CCH参与者的书法技能(包括抄写和创作乐谱)呈正相关。这些结果表明CCH训练改善了某些脑区的部分信息网络拓扑结构。
These results add to the literature on the significant role of structural and functional brain network efficiency in behavior. For example, increased Lp and/or decreased Eloc are often associated with aging [18] and various kinds of brain diseases [19, 20]. Decreased efficiency is often associated with a disrupted network related to brain disease [21, 22].On the other hand, higher efficiency and shorter path length have been linked to a higher intelligence quotient (IQ) [23, 24] in diffusion tensor imaging tractography and RSFC studies [25]. Our study showed that long-term CCH training had positive effects on topological characteristics of the resting-state brain network.
Our finding that the brain regions being affected by CCH training are in the frontal and parietal gyri, limbic system, basal ganglia, and thalamus is consistent our previous analysis with different methods (Chen, et al., 2017). Other forms of art training also seem to share some common effects. For example, CCH and painting training (another form of visual art) would both impact brain areas associated with the executive attention, cognitive control, and motor planning [26]. Musical improvisation has also been correlated with the DMN [27] and long-term musical training with stronger brain functional connectivity between the anterior cingulate cortex, right angular gyrus, and bilateral superior frontal gyrus [28]. However, music training also affects an extensive brain network related to auditory, cognitive, motor, and emotional processing [29], which is different from the CCH-related brain areas. Finally, dancing and piano training has been associated with areas different from those for CCH practice. For example, dancing training is associated with bilateral cerebellum and piano training with the parietal cortex and bilateral cerebellum [30].
As a part of the limbic system, the cingulate cortex plays an important role in emotional processes [31–33]. One recent study found that treatment for anxiety led to higher activation in the cingulate cortex and that the extent of reduction in anxiety was positively correlated with increases in activation [34]. Another study found that the strength of intrinsic connectivity between the PCC and the dorsal attention network was positively correlated with clinical improvements among patients suffering from chronic pain [35]. Emotional processes might have accounted for the effect of CCH training on Eloc of the cingulate cortex because CCH may lower the level of anxiety and lead to stable mood.
In terms of bilateral thalamus that showed shorter Lp and higher Eloc in the CCH group than the control group (as well as significant correlations with better calligraphy skills), it is likely due to the fact that the thalamus is the major center for sensory information processing, including relaying the motor signals. Previous studies have shown that the thalamus plays an important role in the early stages of new learning [36], inhibitory control [37], and motor control [38], all of which are integral to CCH training.
Unlike the cingulate cortex and thalamus, the caudate nucleus showed somewhat inconsistent results (depending on hemisphere, level of analysis [whole brain or modular], and group vs. individual differences). The caudate nucleus is associated with motor process and cognitive functions. For example, activation in the caudate nucleus was greater during spatial and motoric memory tasks than during a nonspatial task [39]. Other studies found that the caudate nucleus showed higher activities in perceptual-motor tasks than in control conditions [40, 41]. Because the hippocampus and the striatum (caudate nucleus and putamen) are two different memory systems involved in place/spatial learning [42, 43], we speculated that these parallel systems might have complicated the relationship between the brain network and calligraphy training.
Finally, the superior medial frontal cortex (SMFC) was correlated with better calligraphy skills. SMFC play a vital role in inhibitory control [44] and the prepotent motor response [45, 46]. Our results underlined the important role of the inhibitory system in the CCH training.
In the current study, we did not find group difference in Module III, which is associated with sensory and motor functions. This result suggests that CCH training does not require more network efficiency than the regular (non-CCH) writing that has to be done by any students. Instead, CCH training seems to affect brain areas for higher cognitive abilities, such as inhibition.
It is also worth mentioning that this study involved quantitative ratings of calligraphy skills, which surprisingly has not been attempted in previous studies of calligraphy. We found that calligraphy skills were, as would be expected, negatively associated with the onset age of CCH practice. It is not clear though whether CCH may have a sensitive period as other kinds of skill acquisition [47, 48].We found that the number of years of CCH practice was correlated with the copying score, but not with the creating score, which is consistent with the common phenomenon that it is relatively easy to learn CCH but quite difficult to reach a mastery level with creative products. We correlated ‘Onset age of practice’ and ‘Years of practice’ with the brain data, but no brain areas survived the multiple correction. This result suggested that CCH level was a better index than were the onset age of practice or years of practice, even though the latter two were also correlated with performance. In other words, it was not how early one started training or how long one was trained, but how well one was trained that made a difference in neural correlates. Finally, although both copying and creating scores were correlated with the DMN, which plays a vital role in the creative process (including artistic creations) [49–52], there were subtle differences in the neural correlates of the two aspects of CCH performance. Whereas copying scores were associated with the LP and Eloc within a relatively widespread network of brain areas, creating scores were correlated with only a few brain areas, mostly the thalamus. Consistent with our results, the thalamus has been linked to creativity [53].
In sum, this study found that long-term CCH training had a positive effect on the efficiency of the resting-state brain network, with visual and DMN-related brain areas showing shorter Lp and higher Eloc for CCH participants than for the controls and with those brain parameters being correlated with better calligraphy skills of CCH participants. However, with a cross-sectional study, the results we found in the current study could not rule out alternative explanatory variables such as training in other art forms (e.g. painting) or personality correlates, nor could we examine potential mediators such as personality traits. Future studies should use a longitudinal design or a randomized training design to examine the causal relations and potential mechanisms.
Supporting information
S1 Fig. Both the CCH and control groups revealed small-world characteristics in their resting-state brain network.
σ = (Cnet/Crand)/(lnet/lrand),σ>1 means the network owns the smallworldness.The two groups showed virtually the same results (overlapping lines), and hence the group x smallworldness interaction was not significant (indicated by empty circles) for any threshold.
(TIF)
Click here for additional data file. (2.1MB, tif)
S2 Fig. The global efficiency of the DMN.
It was significantly higher for the CCH group than the control group at two network thresholds,.16 and.20 (as indicated by the filled circles for the group x efficiency interaction.
(TIF)
Click here for additional data file. (1.3MB, tif)
S1 Table. Brain areas (from the AAL template) of the four modules.
(DOCX)
Click here for additional data file. (23.2KB, docx)
Acknowledgments
This study was supported by the 14YJAZH081 Project of the Ministry of Education of China and the No.31221003 Project of the National Natural Science Foundation of China. We thank all graduate research assistants who helped with data collection.
Data Availability
The correlation matrix data set of resting state brain data for network analyses is available on the Figshare repository (DOI: 10.6084/m9.figshare.7562975, URL: https://figshare.com/s/7f8d94d0e6b9fe819760).
Funding Statement
This study was supported by the 14YJAZH081 (received by WW) Project of the Ministry of Education of the People’s Republic of China (http://en.moe.gov.cn/) and the No.31221003 (received by YH) Project of the National Natural Science Foundation of the People’s Republic of China (http://www.nsfc.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1.Freedberg D, Gallese V. Motion, emotion and empathy in esthetic experience. Trends in Cognitive Sciences. 2007;11(5):197–203. 10.1016/j.tics.2007.02.003 [DOI, click here ] [PubMed, click here ] [Google Scholar]
2.Molnar-Szakacs I, Overy K. Music and mirror neurons: from motion to ’e’motion. Soc Cogn Affect Neur. 2006;1(3):235–41. 10.1093/scan/nsl029 [DOI] [PMC free article] [PubMed] [Google Scholar]
3.Schlaug G, Jancke L, Huang YX, Steinmetz H. In-Vivo Evidence of Structural Brain Asymmetry in Musicians. Science. 1995;267(5198):699–701. 10.1126/science.7839149 [DOI] [PubMed] [Google Scholar]
4.Pantev C, Oostenveld R, Engelien A, Ross B, Roberts LE, Hoke M. Increased auditory cortical representation in musicians. Nature. 1998;392(6678):811–4. 10.1038/33918 [DOI] [PubMed] [Google Scholar]
5.Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus. 2006;16(12):1091–101. 10.1002/hipo.20233 [DOI] [PubMed] [Google Scholar]
6.Boyke J, Driemeyer J, Gaser C, Buechel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28(28):7031–5. 10.1523/JNEUROSCI.0742-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
7.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: Changes in grey matter induced by training—Newly honed juggling skills show up as a transient feature on a brain-imaging scan. Nature. 2004;427(6972):311–2. [DOI] [PubMed] [Google Scholar]
8.Chen W, He Y, Gao Y, Zhang CP, Chen CS, Bi SY, et al. Long-Term Experience of Chinese Calligraphic Handwriting Is Associated with Better Executive Functions and Stronger Resting-State Functional Connectivity in Related Brain Regions. Plos One. 2017;12(1). ARTN e0170660 10.1371/journal.pone.0170660 [DOI] [PMC free article] [PubMed] [Google Scholar]
9.Chen W, Chen CS, He Y, Wang YW, Wang WJ. Long-term Chinese calligraphic handwriting reshapes the cingulate gyrus: a VBM study. Poster presented at: 22nd Annual Meeting of the Organization for the Human Brain Mapping; June 26–30; Geneva, Switzerland2016.
10.Petrella JR. Use of Graph Theory to Evaluate Brain Networks: A Clinical Tool for a Small World? Radiology. 2011;259(2):317–20. 10.1148/radiol.11110380 [DOI] [PubMed] [Google Scholar]
11.Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends in Cognitive Sciences. 2004;8(9):418–25. 10.1016/j.tics.2004.07.008 [DOI] [PubMed] [Google Scholar]
12.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex. 2009;19(1):72–8. 10.1093/cercor/bhn059 [DOI] [PMC free article] [PubMed] [Google Scholar]
13.Akbar N, Giorgio A, Till C, Sled JG, Doesburg SM, De Stefano N, et al. Alterations in Functional and Structural Connectivity in Pediatric-Onset Multiple Sclerosis. Plos One. 2016;11(1). ARTN e0145906 10.1371/journal.pone.0145906 [DOI] [PMC free article] [PubMed] [Google Scholar]
14.Yan CG, Zang YF. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13 Epub 2010/06/26. 10.3389/fnsys.2010.00013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
15.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: A Toolkit for Resting-State Functional Magnetic Resonance Imaging Data Processing. Plos One. 2011;6(9). ARTN e25031 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
16.Kruschwitz JD, List D, Waller L, Rubinov M, Walter H. GraphVar: A user-friendly toolbox for comprehensive graph analyses of functional brain connectivity. J Neurosci Meth. 2015;245:107–15. 10.1016/j.jneumeth.2015.02.021 [DOI] [PubMed] [Google Scholar]
17.Hosseini SMH, Hoeft F, Kesler SR. GAT: A Graph-Theoretical Analysis Toolbox for Analyzing Between-Group Differences in Large-Scale Structural and Functional Brain Networks. Plos One. 2012;7(7). ARTN e40709 10.1371/journal.pone.0040709 [DOI] [PMC free article] [PubMed] [Google Scholar]
18.Gong GL, Rosa P, Carbonell F, Chen ZJ, He Y, Evans AC. Age- and Gender-Related Differences in the Cortical Anatomical Network. J Neurosci. 2009;29(50):15684–93. 10.1523/JNEUROSCI.2308-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
19.He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s Disease. J Neurosci. 2008;28(18):4756–66. 10.1523/JNEUROSCI.0141-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
20.He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132:3366–79. 10.1093/brain/awp089 [DOI] [PMC free article] [PubMed] [Google Scholar]
21.Liao W, Zhang ZQ, Pan ZY, Mantini D, Ding JR, Duan XJ, et al. Altered Functional Connectivity and Small-World in Mesial Temporal Lobe Epilepsy. Plos One. 2010;5(1). ARTN e8525 10.1371/journal.pone.0008525 [DOI] [PMC free article] [PubMed] [Google Scholar]
22.Liu Y, Liang M, Zhou Y, He Y, Hao YH, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–61. 10.1093/brain/awn018 [DOI] [PubMed] [Google Scholar]
23.Gong GL, He Y, Concha L, Lebel C, Gross DW, Evans AC, et al. Mapping Anatomical Connectivity Patterns of Human Cerebral Cortex Using In Vivo Diffusion Tensor Imaging Tractography. Cerebral Cortex. 2009;19(3):524–36. 10.1093/cercor/bhn102 [DOI] [PMC free article] [PubMed] [Google Scholar]
24.Li YH, Liu Y, Li J, Qin W, Li KC, Yu CS, et al. Brain Anatomical Network and Intelligence. Plos Computational Biology. 2009;5(5). ARTN e1000395 10.1371/journal.pcbi.1000395 [DOI] [PMC free article] [PubMed] [Google Scholar]
25.van den Heuvel MP, Stam CJ, Kahn RS, Pol HEH. Efficiency of Functional Brain Networks and Intellectual Performance. J Neurosci. 2009;29(23):7619–24. 10.1523/JNEUROSCI.1443-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
26.Tian F, Chen QL, Zhu WF, Wang YM, Yang WJ, Zhu XX, et al. The association between visual creativity and cortical thickness in healthy adults. Neurosci Lett. 2018;683:104–10. 10.1016/j.neulet.2018.06.036 [DOI] [PubMed] [Google Scholar]
27.Pinho AL, de Manzano O, Fransson P, Eriksson H, Ullen F. Connecting to Create: Expertise in Musical Improvisation Is Associated with Increased Functional Connectivity between Premotor and Prefrontal Areas. J Neurosci. 2014;34(18):6156–63. 10.1523/JNEUROSCI.4769-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
28.Lu J, Yang H, Zhang XX, He H, Luo C, Yao DZ. The Brain Functional State of Music Creation: an fMRI Study of Composers. Scientific Reports. 2015;5 Artn 12277 10.1038/Srep12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
29.Sarkamo T. Music for the ageing brain: Cognitive, emotional, social, and neural benefits of musical leisure activities in stroke and dementia. Dementia-International Journal of Social Research and Practice. 2018;17(6):670–85. 10.1177/1471301217729237 [DOI] [PubMed] [Google Scholar]
30.Lin CS, Liu Y, Huang WY, Lu CF, Teng S, Ju TC, et al. Sculpting the Intrinsic Modular Organization of Spontaneous Brain Activity by Art. Plos One. 2013;8(6). ARTN e66761 10.1371/journal.pone.0066761 [DOI] [PMC free article] [PubMed] [Google Scholar]
31.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–22. 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
32.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–48. 10.1006/nimg.2002.1087 [DOI] [PubMed] [Google Scholar]
33.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
34.Burkhouse KL, Kujawa A, Hosseini B, Klumpp H, Fitzgerald KD, Langenecker SA, et al. Anterior cingulate activation to implicit threat before and after treatment for pediatric anxiety disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2018;84:250–6. 10.1016/j.pnpbp.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
35.Yoshino A, Okamoto Y, Okada G, Takamura M, Ichikawa N, Shibasaki C, et al. Changes in resting-state brain networks after cognitive-behavioral therapy for chronic pain. Psychological Medicine. 2018;48(7):1148–56. 10.1017/S0033291717002598 [DOI] [PubMed] [Google Scholar]
36.Winocur G. The Hippocampus and Thalamus—Their Roles in Short-Term and Long-Term-Memory and the Effects of Interference. Behavioural Brain Research. 1985;16(2–3):135–52. 10.1016/0166-4328(85)90088-9 [DOI] [PubMed] [Google Scholar]
37.Albin RL, Young AB, Penney JB. The Functional-Anatomy of Basal Ganglia Disorders. Trends in Neurosciences. 1989;12(10):366–75. 10.1016/0166-2236(89)90074-X [DOI] [PubMed] [Google Scholar]
38.Chang WH, Kim YH, Yoo WK, Goo KH, Park CH, Kim ST, et al. rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restorative Neurology and Neuroscience. 2012;30(3):179–89. 10.3233/RNN-2012-110162 [DOI] [PMC free article] [PubMed] [Google Scholar]
39.Postle BR, M D’Esposito. Dissociation of human caudate nucleus activity in spatial and nonspatial working memory: an event-related fMRI study. Cognitive Brain Res. 1999;8(2):107–15. 10.1016/S0926-6410(99)00010-5 [DOI] [PubMed] [Google Scholar]
40.Schwabe L, Tegenthoff M, Hoffken O, Wolf OT. Mineralocorticoid Receptor Blockade Prevents Stress-Induced Modulation of Multiple Memory Systems in the Human Brain. Biological Psychiatry. 2013;74(11):801–8. 10.1016/j.biopsych.2013.06.001 [DOI] [PubMed] [Google Scholar]
41.Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J Neurosci. 2003;23(13):5945–52. 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
42.Mcdonald RJ, White NM. Parallel Information-Processing in the Water Maze—Evidence for Independent Memory-Systems Involving Dorsal Striatum and Hippocampus. Behavioral and Neural Biology. 1994;61(3):260–70. 10.1016/S0163-1047(05)80009-3 [DOI] [PubMed] [Google Scholar]
43.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiology of Learning and Memory. 2002;77(2):125–84. 10.1006/nlme.2001.4008 [DOI] [PubMed] [Google Scholar]
44.Bender AD, Filmer HL, Dux PE. Transcranial direct current stimulation of superior medial frontal cortex disrupts response selection during proactive response inhibition. Neuroimage. 2017;158:455–65. Epub 2016/10/30. 10.1016/j.neuroimage.2016.10.035 . [DOI] [PubMed] [Google Scholar]
45.Chen CY, Muggleton NG, Tzeng OJL, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. Neuroimage. 2009;44(2):537–45. 10.1016/j.neuroimage.2008.09.005 [DOI] [PubMed] [Google Scholar]
46.Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. Journal of Cognitive Neuroscience. 2006;18(11):1843–9. 10.1162/jocn.2006.18.11.1843 [DOI] [PubMed] [Google Scholar]
47.Hannon EE, Trehub SE. Tuning in to musical rhythms: Infants learn more readily than adults. P Natl Acad Sci USA. 2005;102(35):12639–43. 10.1073/pnas.0504254102 [DOI] [PMC free article] [PubMed] [Google Scholar]
48.Steele CJ, Bailey JA, Zatorre RJ, Penhune VB. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(3):1282–90. Epub 2013/01/18. 10.1523/JNEUROSCI.3578-12.2013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
49.Beaty RE, Benedek M, Silvia PJ, Schacter DL. Creative Cognition and Brain Network Dynamics. Trends in Cognitive Sciences. 2016;20(2):87–95. 10.1016/j.tics.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
50.Beaty RE, Kenett YN, Christensen AP, Rosenberg MD, Benedek M, Chen QL, et al. Robust prediction of individual creative ability from brain functional connectivity. P Natl Acad Sci USA. 2018;115(5):1087–92. 10.1073/pnas.1713532115 [DOI] [PMC free article] [PubMed] [Google Scholar]
51.Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. Neuroimage. 2012;59(2):1783–94. 10.1016/j.neuroimage.2011.08.008 [DOI] [PubMed] [Google Scholar]
52.Min BK. A thalamic reticular networking model of consciousness. Theoretical Biology and Medical Modelling. 2010;7 Artn 10 10.1186/1742-4682-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
53.de Manzano O, Cervenka S, Karabanov A, Farde L, Ullen F. Thinking Outside a Less Intact Box: Thalamic Dopamine D2 Receptor Densities Are Negatively Related to Psychometric Creativity in Healthy Individuals. Plos One. 2010;5(5). ARTN e10670 10.1371/journal.pone.0010670 [DOI] [PMC free article] [PubMed] [Google Scho
The Main Styles of Chinese Calligraphy
汉字书法的主要风格
Calligraphy (書法 shū fǎ) is, along with ink painting, with which it is closely related, one of the most practiced ancient arts in China. Scholars, poets and painters were often great calligraphers. This art is regarded not only as an aesthetic form of writing but also as a way to cultivate one's own character and to develop gōng fu 功夫.
书法(shū fǎ)与其密切相关的水墨画是中国最古老的艺术之一。学者、诗人和画家往往都是伟大的书法家。书法不仅被视为一种审美的书写形式,也被视为一种陶冶情操和培养功夫的方式。
There are five main styles of Chinese calligraphy. These are, following the chronological order of appearance: Seal Script (篆书 zhuàn shū), Clerical Script (隸書 lì shū), Cursive Script (草書 cǎo shū), Semi-Cursive Script (行書 xíng shū) and Standard Script (楷書 kǎi shū). It is worth mentioning, although it is not a style of calligraphy, the Oracule Bone Script (甲骨文 jiǎ gǔ wén), as the first form of writing to appear in China.
Oracle Bone Script 甲骨文 jiǎ gǔ wén
Oracle Bone Script is the earliest known form of writing in China, appeared between the 14th and 11th centuries BC. It was practiced in the form of pictographic inscriptions on animal bones and turtle shells, for divinatory purposes. This type of writing was discovered and deciphered in the early 20th century. It is characterized by its strokes that begin and end abruptly, due to the hardness of both the materials on which they are inscribed, and those with which the incisions were practiced, such as knives or needles.
Caligrafía china, Gong Fu, estilos de caligrafía, huesos oraculares
Caligrafía china, Gong Fu, estilos de caligrafía, huesos oraculares
Caligrafía china, Gong Fu, estilos de caligrafía, huesos oraculares
Caligrafía china, Gong Fu, estilos de caligrafía, huesos oraculares
Bone and turtle plastron with oracular inscriptions. Museum of the Mausoleum of the Nányuè 南越 King, Guangzhou 广州.
Seal Script 篆書 zhuàn shū
As a form of writing, it appears during the Qín 秦 dynasty (221-206 BC), and represents an evolution from Oracle Bone Script. It uses seals inscribed on jade or ritual bronze vessels. Similar to bone inscriptions, its strokes are thin and sharp at the ends and do not vary in width, because they are inscribed on hard materials.
This form of writing is divided into Dà Zhuàn 大篆 (Great Seal), which is derived from the archaic characters of the Oracle Script, and Xiǎo Zhuàn 小篆 (Small Seal), which simplifies the characters by removing pictographic elements. The Xiǎo Zhuàn became the official form of writing after the unification of the language during the Qín dynasty.
Later, this kind of script reemerged as a form of artistic expression and is considered one of the main styles of calligraphy. Today it is practiced with brush and is characterized by its solid characters, larger in height than in width and devoid of emotion, with symmetry both in the execution of the strokes and in their arrangement, as well as by the careful layout of the white space between strokes. To achieve symmetry in the lines, the tip of the brush must be kept in the center, without falling sideways. Writing a character in this style takes between one and two minutes.
It is also used to sign writings and paintings, with stone stamps printed on paper with red ink.
Escritura Sellos - The Main Styles of Chinese Calligraphy
Seal Script Xiǎo Zhuàn
Escritura de Sellos - The Main Styles of Chinese Calligraphy
Ancient dictionary from Hàn dynasty that analyses the structure of Seal Script
Clerical Script 隸書 lì shū
At the end of the first millennium BC use of ink spread; this favoured the appearance of a new style of writing, practiced with a brush on bamboo or wooden strips (简牍 jiǎndú) sewn between them in the form of rolls. In use throughout the entire Hàn dynasty, it was mainly used by government personnel to draft official documents.
Tablillas de bambu - The Main Styles of Chinese Calligraphy
Remains of an old wooden strip roll used to write documents.
It was during this time that calligraphy began to be regarded as an art and not only as a mere form of communication. The flexibility of the new tools used, brushes made of animal hair, made it possible to modulate the strokes at will, so these began to vary in width. The techniques of tí 提 and àn 按 were developed, consisting respectively of lifting and pressing the brush to modify the thickness of the line.
This style of writing follows strict rules. Its most characteristic stroke is the so-called 'silkworm head and wild goose tail', which consists of a horizontal line that begins on the left with a rounded shape and ends on the right with the tip facing up. However, with few exceptions, only one of the horizontal lines in each word will take this form, following the rule "two goose tails do not fly together". Another feature of lì shū is the justification of all the characters on the top line.
Cabeza de Gusano y Cola de Ganso - The Main Styles of Chinese Calligraphy
The character 生 shēng, 'life', written on lì shū 隸書 script.
Its horizontal line at the bottom uses the 'silkworm head and wild goose tail' stroke.
The Chinese name of this style, lì shū, “servant script”, is due to the fact that its invention is attributed to a prison officer.
Escritura Administrativa - The Main Styles of Chinese Calligraphy
Clerical Scrpit lì shū 隸書.
Cursive or Running Script 草書 cǎo shū
This style of calligraphy originated at the end of Hàn dynasty and continued to develop until the middle of Táng 唐朝 dynasty (618-907), as a consequence of the aesthetic potential of writing with ink on paper.
Its main feature is the merging of the different strokes, which in other styles have to be executed separately, into a single and continuous movement of the brush. The characters take on a rounded, angleless shape, and resemble the grass shaked by the wind, so this style is referred to as "Grass Script" in China. In addition to merging the strokes, the different characters are linked together and are written without lifting the brush from the paper. Its writing is quick but its reading is difficult to the person unfamiliar with the former. It allows more freedom in the production of writing and in the expression of emotions, being possible to deform the characters, proportions and thickness of the lines, although there are still rules for its production.
Escritura Cursiva - The Main Styles of Chinese Calligraphy
Cursive Script 草書 cǎo shū
Semi-Cursive Script 行書 xíng shū
This style of calligraphy appeared after the Hàn dynasty, but reached its maximum development before the Cursive Script did. It is characterized by being smoother and faster than Standard Script but less than Cursive.
Semi-Cursive Script became the most popular form of handwriting, and remains the most widely used today.
Caligrafia Semi Cursiva - The Main Styles of Chinese Calligraphy
Semi-Cursive Script 行書 xíng shū
Standard Script 楷書 kǎi shū
Standard Script was developed in parallel with the last two styles described, as a result of combining the separate strokes of Clerical Script with those more fluid and asymmetrical of Cursive Script. It is therefore characterized by containing the largest number of strokes, executed separately, slowly and carefully, following stricter rules regarding their width and proportions. It is the first style taught to children, and is the basis for almost all modern printed materials, as it is the clearest and easiest-to-read form of calligraphy.
Escritura Estandar - The Main Styles of Chinese Calligraphy
Standard Script 楷書 kǎi shū
Although all these forms of callgraphy had already developed entirely in the Táng dynasty, calligraphy as an art has continued to develop over the centuries to the present day.
Los Cinco Estilos de Caligrafia - The Main Styles of Chinese CalligraphyThe words 書 shū, 'script' (top), and 道 dào, 'way' (bottom), according to the main five styles of Chinese calligraphy.
From left to right: Seal, Clerical, Standard, Semi-Cursive and Cursive Script.
十年树木,百年树人
"Ten years to cultivate wood,
a hundred years to cultivate a person's character"
Chinese proverb
Gwong Zau Kung Fu 廣州功夫
Gong Fu and Chinese Culture
Learn more: PMC Disclaimer | PMC Copyright Notice
Abstract
As a visual art form, Chinese calligraphic handwriting (CCH) has been found to correlate with certain brain activity and to induce functional connectivity reorganization of the brain. This study investigated the effect of long-term CCH training on brain functional plasticity as assessed with network measures. With the resting-state fMRI data from 31 participants with at least five years of CCH training and 40 controls, we constructed brain functional networks, examined group differences at both the whole brain and modular levels, and correlated the topological characteristics with calligraphy skills. We found that, compared to the control group, the CCH group showed shorter characteristic path lengths and higher local efficiency in certain brain areas in the frontal and parietal cortices, limbic system, basal ganglia, and thalamus. Moreover, these network measures in the cingulate cortex, caudate nucleus, and thalamus were associated with CCH performance (i.e., copying and creating skills). These results suggest that long-term CCH training has a positive effect on the topological characteristics of brain networks.
1. Introduction
Chinese calligraphic handwriting (CCH) is a 3000-year-old art form. To master CCH skills requires years of intensive practice that involves sensory perception, motor skills, as well as multiple cognitive and emotional elements [1, 2]. Following previous research that found both structural and functional brain plasticity in response to many types of intensive training such as musical training [3, 4], driving [5], and juggling [6, 7], we have examined brain plasticity related to CCH training. Our previous two studies found that CCH training strengthened the RSFC of brain areas involved in updating and inhibition [8] and decreased the volume of the posterior cingulate cortex (PCC) [9].
In addition to the traditional univariate neuroimaging methods such as voxel-based morphometry (VBM) and resting-state functional connectivity (RSFC) used in the studies mentioned above, researchers have recently paid attention to brain connectivity networks or modular organization. Brain network analysis can mathematically describe various topological parameters of the brain’s organization in terms of graphs or networks, including the small-worldness, modularity, and regional network parameters [10, 11]. Studies have proved that functionally connected resting-state brain networks are associated with the anatomical connectivity of the brain [12, 13].
Given our previous findings of CCH training’s effects on the RSFC of certain brain areas [8], we hypothesized long-term CCH practicing would have an effect on the topological parameters of the resting-state brain network, including the frontal and parietal cortices, basal ganglia, and PCC. We explored the long-term CCH training’s effect on the topological characteristics of the whole brain and four specific modules. These modules were selected because of their relevance to visual processing (Module I), sensorimotor functions (Module II), and DMN (Module III), all of which are involved in CCH. More details of the brain areas included in each module are shown Fig 1 and S1 Table. Finally, within the CCH group, we further investigated the relationship between global and local network measures and calligraphy skills.
Fig 1. Visualization of the four modules selected for network efficiency analyses.
Modules I, II, and III mean the sets of brain areas involved in visual processing, sensorimotor functions, and the DMN, respectively. L: left hemisphere; R: right hemisphere.
2. Materials and methods
2.1. Participants
Participants were recruited from Beijing Normal University, Beijing, China. The CCH group included 32 students who majored in calligraphy and had at least five years of formal training in CCH and the control group included 44 students who had no more than a few months of basic CCH skill training. All subjects were right-handed native Chinese speakers. Participants’ IQ was measured with Raven’s Advanced Progressive Matrices (APM) (for details, see Chen et al., 2017) [8]. Each participant signed an informed consent form after a full explanation of the study procedure. This study was approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neuroscience and Learning at Beijing Normal University, China. Subjects were compensated for their time.
2.2 Calligraphy skills
Assessment of participants’ calligraphy skills was based on their performance on two tasks: copying a famous calligraphy work and creating a new calligraphy work.
All participants of the CCH group were asked to copy a part of a calligraphy masterpiece titled “the Northern Wei sculpture’ and to create a calligraphy work (in any style) using an existing quatrain written by Qi Gong, a celebrated cultural figure in China.
Participants’ works (with identity information removed) were evaluated by seven calligraphy teachers from the Department of Calligraphy of the School of Arts and Media, Beijing Normal University. They used a 10-point scoring system (from 1 to 10 with interval of 0.5). The final score for each piece of work was the mean of the five non-extreme scores after eliminating two extreme scores (the highest and the lowest) (see the Results section for interjudge reliability).
2.3 Brain imaging data collection and preprocessing
2.3.1 fMRI data acquisition
All scanning was performed using a SIEMENS TRIO 3-Tesla scanner in the Brain Imaging Center of Beijing Normal University. Participants were told neither to have heavy physical activities nor have stimulating drinks the day before the scanning. Each participant underwent a 3D anatomic session and an eight-minute resting-state fMRI (RS-fMRI) scanning session. The 3D T1-weighted magnetization- prepared rapid gradient echo (MPRAGE) image was acquired with the following parameters: 144 sagital slices, slice thickness/gap = 1.3/0.65 mm, TR = 2530 ms, TE = 3.39 ms, inversion time (Ti) = 1100 ms, flip angle = 7°, FOV = 256×256 mm2, matrix size = 256×192. During the RS-fMRI session, the participants were instructed to keep their eyes closed, be as still as possible, and not to think about anything in particular. Images were obtained with the following parameters: 33 axial slices, thickness/gap = 3.5/0.7 mm, matrix size = 64×64, repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 200×200 mm2.
2.3.2 Image preprocessing and analysis
Resting-State fMRI data were preprocessed using DPARSE (DPARSF, http://rfmri.org/DPARSF) [14]. Briefly, after discarding the first 10 volumes, the following steps were performed: correction, coregistration, segmentation, normalization, smoothing, linear detrending, regressing nuisance signals, and filtering. For more detail, see Chen et al. (2017).
In order to construct the connectivity network, the preprocessed RS-fMRI images data were overlapped with ALL templates (90 cortical and 26 cerebellar anatomical areas), and then the mean time series of all the 116 nodes were calculated in REST [15]. Pearson correlation was performed to obtain ‘r-value matrix’ and then Fisher’s r-to-z transformation was employed to obtain ‘z-score matrix’.
2.4 Statistics
After obtaining the correlation matrix, we used GraphVar (http://rfmri.org/GraphVar), a GUI-based toolbox for graph theoretical methods [16], to calculate the global and local clustering coefficient (Cp), global and local characteristic path length (Lp), global efficiency (Eglob), and local efficiency (Eloc) of the whole gray matter brain (90 ALL brain areas) and four modules with a range of network cost (0.1~0.5). We also generated 100 binary random networks per subject per threshold (null-model networks) to test the difference between network measures and the random networks, and also tested the small-worldness trait of human brain resting-state network. With age, gender and IQ as covariates, we then performed group analyses with 1000 permutations (corrected p <.05), which is a non-parametric testing method to detect the statistical significance of the group differences in network topological characteristics [16, 17]. The analyses were conducted both for the whole brain and the four modules.
Within the CCH group, we also correlated calligraphy skills (copying scores and creating scores) with topological characteristics (global and local Cp, Lp, Eglob and Eloc).
3. Results
3.1 Reliability analysis of the calligraphy skills
We used Kendall’s coefficient of concordance to assess the agreement among the seven judges of the calligraphy skills. The Kendall’s W value was 0.719 (p< 0.001) for copying skills and 0.800 (p< 0.001) for creating skills. Because four of the seven judges were the participants’ teachers, we correlated the average ratings of the four calligraphy teachers of the participants and those of the three judges who had not taught these participants. Results showed very high correlations between them for the two measures of quality of CCH: the coping scores (r =.882, p <.01) and creating scores (r =.863, p <.01). These results suggest that even if the teachers might have been able to infer the identities of the participants from the anonymized writings, they were making similar judgments as the blind judges.
3.2 Demographic data
There was no group difference between the CCH and control groups in terms of gender, age (CCH group: 21.23±2.11; control group: 21.61±2.54), years of education (CCH group: 14.34±2.04; control group: 14.59±1.85), and IQ (CCH group: 26.47±3.68; control group: 27.58±3.74). The CCH practitioners had an average of 10 years of experience and started practicing at around 9 years of age (for more details, see Chen et. al, (2017).
Copying score was correlated negatively with the onset age of CCH practice (r = -0.627, p<0.001) and positively with the number of years of practice (r = 0.390, p = 0.021). Creating skills were significantly correlated with only the onset age of practice (r = -0.347, p = 0.041) (Table 1).
Table 1. The relationship between the time factors of CCH practice and calligraphy skills.
3.3 Topological characteristics of the RSFC networks
3.3.1 Whole brain network
Six topological properties, the global Cp and Lp, local Cp and Lp, Eglob and Eloc, were used to characterize the global and local topological properties of the brain network. The two groups did not differ in small-world characteristics of brain organization (S1 Fig). They also did not differ in global Cp, Lp and Eglob. For the local parameters, we found that compared to the control group, the CCH group showed larger local Cp in right middle frontal gyrus, left inferior triangle frontal gyrus, left anterior and median cingulum, right hippocampus gyrus, right inferior frontal gyrus, right anterior cingulum, right calcarine fissure, and right fusiform gyrus (Table 2). The CCH group also showed shorter local Lp in right anteriror cingulum, left supramarginal gyrus, left caudate nucleus, and bilateral thalamus, but longer Lp in right hippocampus and caudate nucleus (Table 3). Finally, the CCH group showed higher Eloc in right PCC, right superior and inferior parietal gyrus, caudate nucleus, and thalamus (Table 4).
Table 2. Brain regions with significant group differences in characteristic path length between the CCH and control groups (corrected p <.05).
Note: Positive t values mean that the CCH group had longer paths, whereas negative t values mean that the control group had longer paths.
Table 3. Brain regions with significant group differences in local clustering coefficient between the CCH and control groups (corrected p <.05).
Note: Positive t values mean that the CCH group had larger coefficients, whereas negative t values mean that the control group had larger coefficients.
Table 4. Brain regions with significant differences in local efficiency between the CCH and control groups (corrected p <.05).
Note: Positive t values mean that the CCH group had higher local efficiency, whereas negative t values mean that the control group had higher local efficiency.
3.3.2 Group difference in modular network efficiency
We chose four modular networks that are most relevant to CCH training for the network efficiency analysis. Module I (visual processing) showed that the CCH group had high lower Cp in right calcarine fissure and higher Cp in left MOG than did the control group (corrected alpha level of 0.05 with 1000 permutations, at the threshold of 0.19–0.45). The CCH group also showed longer Lp in right inferior occipital gyrus and shorter Lp in left fusiform. The CCH group showed higher Eloc in bilateral middle occipital gyrus (Fig 2). Finally, no group difference was found in terms of Eglobal in Module I.
Fig 2. Bilateral middle occipital region showed higher Eloc in the CCH group than in the control group (red) in Module I, with 1000 permutations (p <.05).
Note: 1. Calcarine_L; 2. Calcarine_R; 3. Cuneus_L; 4. Cuneus_R; 5. Lingual_L; 6. Lingual_R; 7. Occipital_Sup_L; 8. Occipital_Sup_R; 9. Occipital_Mid_L; 10. Occipital_Mid_R; 11. Occipital_Inf_L; 12. Occipital_Inf_R; 13. Fusiform_L; 14. Fusiform_R.
Contrary to our expectations, Module II (the sensorimotor areas) showed no significant group differences. For Module III (the DMN), the CCH group showed higher Cp in left superior and medial frontal gyrus and lower Cp in bilateral precuneus than did the control group. The CCH group also showed significantly shorter Lp when the network threshold was 0.15, 0.16, 0.19 and 0.20. At the local level, the CCH group showed significantly shorter Lp than the control group in left anterior cingulum and bilateral precuneus, but higher Lp in right caudate nucleus. In contrast, the CCH group showed significantly higher Eloc in bilateral precuneus, and lower Eloc in bilateral caudate nucleus (Fig 3). The CCH group tended to have higher Eglobal than the control group, which reached significance at two network thresholds (0.16 and 0.20) (S2 Fig).
Fig 3. The CCH group showed higher Eloc in bilateral precuneus (red) and lower in bilateral caudate nucleus (green) than the control group in Module III.
Note: 1. Frontal_Sup_L (dorsolateral); 2. Frontal_Sup_R (dorsolateral); 3. Frontal_Mid_L; 4. Frontal_Mid_R; 5. Frontal_Mid_Orb_L; 6. Frontal_Sup_Medial_L; 7. Frontal_Sup_Medial_R; 8. Cingulum_Ant_L (Anterior cingulate and paracingulate gyri); 9. Angular_L; 10. Angular_R; 11. Precuneus_L; 12. Precuneus_R; 13. Caudate_L; 14. Caudate_R; 15. Thalamus_L; 16.Thalamus_R.
3.4 Correlation analyses between brain network parameters and calligraphy skills
The above results showed group differences in topological characteristics (shorter Lp and higher Eloc for the CCH group than the control group). To extend those results to individual differences within the CCH group, we conducted correlation analyses between brain network parameters and calligraphy skills. The correlational analyses generally confirmed those associations within the CCH group. Specifically, within thresholds 0.13~0.21, copying score was negatively correlated with characteristic path length at the global level. At the local level, the Lp of certain brain regions (i.e., bilateral olfactory cortex, amygdala, caudate nucleus; right rectus, thalamus and middle temporal pole) all showed negative relationship with copying score, whereas the Lp of left thalamus was negatively correlated with creating score and the Lp of left inferior occipital gyrus and bilateral caudate nucleus was positively correlated with creating score.
Cp in the right supplementary motor areas, right superior occipital gyrus, and left inferior occipital gyrus was negatively correlated with copying scores, but Cp in right gyrus rectus and amygdala was positively correlated with copying score. Cp in the right calcarine fissure, cuneus, superior and middle occipital gyrus, middle temporal gyrus, and left inferior occipital gyrus was negatively correlated with creating score, and that of right inferior frontal gyrus was positively correlated with creating score.
The Eloc of bilateral superior medial frontal gyrus and PCC, right hippocampus and lenticular nucleus (pallidum), and left temporal pole (middle temporal gyrus) showed positive relationship with copying score. The Eloc of left supplementary motor area, insula, bilateral paracentral lobule, caudate nucleus and thalamus, and right lenticular nucleus (pallidum) was positively correlated with creating score, but Eloc of left inferior occipital gyrus was negatively correlated with creating score (Table 5).
Table 5. The positive (+) and negative (-) correlation brain regions with r values between local topological characteristics (i. e., Lp, Cp, Eloc) and calligraphy skills.
Note: + positive correlation (P < 0.05),
− negative correlation (P < 0.05),
N no significant correlation brain regions (P > 0.05).
Taken together, we found that the cingulate cortex, caudate nucleus and thalamus were the core brain areas that showed both group differences between the CCH and control groups and significant correlations with calligraphy skills within the CCH group (Table 6).
Table 6. Group differences and neural correlates (within the DMN) of CCH skills.
+ means the CCH group had longer Lp/higher Eloc than the control group or positive correlation.
− means the CCH group had shorter Lp/lower Eloc than the control group or negative correlation (P < 0.05).
4. Discussion
The current study explored the effect the long-term experience with CCH on brain network efficiency assessed with parameters based on graph theory. We found that compared to the controls, individuals with long-term CCH training showed advantages in topological characteristics (i.e., Lp, Cp and Eloc) in certain brain areas based on both whole brain and modular analyses. Moreover, within the CCH group, calligraphy skills were associated with brain network efficiency parameters, especially Lp and Eloc.
Seven brain regions showed significant group differences in Lp (Table 3), with the CCH group having shorter Lp than the control group for five of the seven regions. It appears that CCH training increased the information transfer speed (as indexed by Lp from one brain area to another). Consistent with the group differences, copying score had a significant negative relationship with Lp within CCH participants. Similar to the results with Lp, the CCH group had higher Eloc than the control group in all brain areas in the right hemisphere and Eloc in many of these brain regions was positively correlated with calligraphy skills (including both copying and creating scores) of CCH participants. Those results demonstrated that CCH training improved the partial information network’s topological structure of certain brain areas.
These results add to the literature on the significant role of structural and functional brain network efficiency in behavior. For example, increased Lp and/or decreased Eloc are often associated with aging [18] and various kinds of brain diseases [19, 20]. Decreased efficiency is often associated with a disrupted network related to brain disease [21, 22].On the other hand, higher efficiency and shorter path length have been linked to a higher intelligence quotient (IQ) [23, 24] in diffusion tensor imaging tractography and RSFC studies [25]. Our study showed that long-term CCH training had positive effects on topological characteristics of the resting-state brain network.
Our finding that the brain regions being affected by CCH training are in the frontal and parietal gyri, limbic system, basal ganglia, and thalamus is consistent our previous analysis with different methods (Chen, et al., 2017). Other forms of art training also seem to share some common effects. For example, CCH and painting training (another form of visual art) would both impact brain areas associated with the executive attention, cognitive control, and motor planning [26]. Musical improvisation has also been correlated with the DMN [27] and long-term musical training with stronger brain functional connectivity between the anterior cingulate cortex, right angular gyrus, and bilateral superior frontal gyrus [28]. However, music training also affects an extensive brain network related to auditory, cognitive, motor, and emotional processing [29], which is different from the CCH-related brain areas. Finally, dancing and piano training has been associated with areas different from those for CCH practice. For example, dancing training is associated with bilateral cerebellum and piano training with the parietal cortex and bilateral cerebellum [30].
As a part of the limbic system, the cingulate cortex plays an important role in emotional processes [31–33]. One recent study found that treatment for anxiety led to higher activation in the cingulate cortex and that the extent of reduction in anxiety was positively correlated with increases in activation [34]. Another study found that the strength of intrinsic connectivity between the PCC and the dorsal attention network was positively correlated with clinical improvements among patients suffering from chronic pain [35]. Emotional processes might have accounted for the effect of CCH training on Eloc of the cingulate cortex because CCH may lower the level of anxiety and lead to stable mood.
In terms of bilateral thalamus that showed shorter Lp and higher Eloc in the CCH group than the control group (as well as significant correlations with better calligraphy skills), it is likely due to the fact that the thalamus is the major center for sensory information processing, including relaying the motor signals. Previous studies have shown that the thalamus plays an important role in the early stages of new learning [36], inhibitory control [37], and motor control [38], all of which are integral to CCH training.
Unlike the cingulate cortex and thalamus, the caudate nucleus showed somewhat inconsistent results (depending on hemisphere, level of analysis [whole brain or modular], and group vs. individual differences). The caudate nucleus is associated with motor process and cognitive functions. For example, activation in the caudate nucleus was greater during spatial and motoric memory tasks than during a nonspatial task [39]. Other studies found that the caudate nucleus showed higher activities in perceptual-motor tasks than in control conditions [40, 41]. Because the hippocampus and the striatum (caudate nucleus and putamen) are two different memory systems involved in place/spatial learning [42, 43], we speculated that these parallel systems might have complicated the relationship between the brain network and calligraphy training.
Finally, the superior medial frontal cortex (SMFC) was correlated with better calligraphy skills. SMFC play a vital role in inhibitory control [44] and the prepotent motor response [45, 46]. Our results underlined the important role of the inhibitory system in the CCH training.
In the current study, we did not find group difference in Module III, which is associated with sensory and motor functions. This result suggests that CCH training does not require more network efficiency than the regular (non-CCH) writing that has to be done by any students. Instead, CCH training seems to affect brain areas for higher cognitive abilities, such as inhibition.
It is also worth mentioning that this study involved quantitative ratings of calligraphy skills, which surprisingly has not been attempted in previous studies of calligraphy. We found that calligraphy skills were, as would be expected, negatively associated with the onset age of CCH practice. It is not clear though whether CCH may have a sensitive period as other kinds of skill acquisition [47, 48].We found that the number of years of CCH practice was correlated with the copying score, but not with the creating score, which is consistent with the common phenomenon that it is relatively easy to learn CCH but quite difficult to reach a mastery level with creative products. We correlated ‘Onset age of practice’ and ‘Years of practice’ with the brain data, but no brain areas survived the multiple correction. This result suggested that CCH level was a better index than were the onset age of practice or years of practice, even though the latter two were also correlated with performance. In other words, it was not how early one started training or how long one was trained, but how well one was trained that made a difference in neural correlates. Finally, although both copying and creating scores were correlated with the DMN, which plays a vital role in the creative process (including artistic creations) [49–52], there were subtle differences in the neural correlates of the two aspects of CCH performance. Whereas copying scores were associated with the LP and Eloc within a relatively widespread network of brain areas, creating scores were correlated with only a few brain areas, mostly the thalamus. Consistent with our results, the thalamus has been linked to creativity [53].
In sum, this study found that long-term CCH training had a positive effect on the efficiency of the resting-state brain network, with visual and DMN-related brain areas showing shorter Lp and higher Eloc for CCH participants than for the controls and with those brain parameters being correlated with better calligraphy skills of CCH participants. However, with a cross-sectional study, the results we found in the current study could not rule out alternative explanatory variables such as training in other art forms (e.g. painting) or personality correlates, nor could we examine potential mediators such as personality traits. Future studies should use a longitudinal design or a randomized training design to examine the causal relations and potential mechanisms.
Supporting information
σ = (Cnet/Crand)/(lnet/lrand),σ>1 means the network owns the smallworldness.The two groups showed virtually the same results (overlapping lines), and hence the group x smallworldness interaction was not significant (indicated by empty circles) for any threshold.
(TIF)
It was significantly higher for the CCH group than the control group at two network thresholds,.16 and.20 (as indicated by the filled circles for the group x efficiency interaction.
(TIF)
(DOCX)
Acknowledgments
This study was supported by the 14YJAZH081 Project of the Ministry of Education of China and the No.31221003 Project of the National Natural Science Foundation of China. We thank all graduate research assistants who helped with data collection.
Data Availability
The correlation matrix data set of resting state brain data for network analyses is available on the Figshare repository (DOI: 10.6084/m9.figshare.7562975, URL: https://figshare.com/s/7f8d94d0e6b9fe819760).
Funding Statement
This study was supported by the 14YJAZH081 (received by WW) Project of the Ministry of Education of the People’s Republic of China (http://en.moe.gov.cn/) and the No.31221003 (received by YH) Project of the National Natural Science Foundation of the People’s Republic of China (http://www.nsfc.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Freedberg D, Gallese V. Motion, emotion and empathy in esthetic experience. Trends in Cognitive Sciences. 2007;11(5):197–203. 10.1016/j.tics.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 2.Molnar-Szakacs I, Overy K. Music and mirror neurons: from motion to ’e’motion. Soc Cogn Affect Neur. 2006;1(3):235–41. 10.1093/scan/nsl029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlaug G, Jancke L, Huang YX, Steinmetz H. In-Vivo Evidence of Structural Brain Asymmetry in Musicians. Science. 1995;267(5198):699–701. 10.1126/science.7839149 [DOI] [PubMed] [Google Scholar]
- 4.Pantev C, Oostenveld R, Engelien A, Ross B, Roberts LE, Hoke M. Increased auditory cortical representation in musicians. Nature. 1998;392(6678):811–4. 10.1038/33918 [DOI] [PubMed] [Google Scholar]
- 5.Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus. 2006;16(12):1091–101. 10.1002/hipo.20233 [DOI] [PubMed] [Google Scholar]
- 6.Boyke J, Driemeyer J, Gaser C, Buechel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28(28):7031–5. 10.1523/JNEUROSCI.0742-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: Changes in grey matter induced by training—Newly honed juggling skills show up as a transient feature on a brain-imaging scan. Nature. 2004;427(6972):311–2. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, He Y, Gao Y, Zhang CP, Chen CS, Bi SY, et al. Long-Term Experience of Chinese Calligraphic Handwriting Is Associated with Better Executive Functions and Stronger Resting-State Functional Connectivity in Related Brain Regions. Plos One. 2017;12(1). ARTN e0170660 10.1371/journal.pone.0170660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Chen CS, He Y, Wang YW, Wang WJ. Long-term Chinese calligraphic handwriting reshapes the cingulate gyrus: a VBM study. Poster presented at: 22nd Annual Meeting of the Organization for the Human Brain Mapping; June 26–30; Geneva, Switzerland2016.
- 10.Petrella JR. Use of Graph Theory to Evaluate Brain Networks: A Clinical Tool for a Small World? Radiology. 2011;259(2):317–20. 10.1148/radiol.11110380 [DOI] [PubMed] [Google Scholar]
- 11.Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends in Cognitive Sciences. 2004;8(9):418–25. 10.1016/j.tics.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 12.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex. 2009;19(1):72–8. 10.1093/cercor/bhn059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akbar N, Giorgio A, Till C, Sled JG, Doesburg SM, De Stefano N, et al. Alterations in Functional and Structural Connectivity in Pediatric-Onset Multiple Sclerosis. Plos One. 2016;11(1). ARTN e0145906 10.1371/journal.pone.0145906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan CG, Zang YF. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010;4:13 Epub 2010/06/26. 10.3389/fnsys.2010.00013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: A Toolkit for Resting-State Functional Magnetic Resonance Imaging Data Processing. Plos One. 2011;6(9). ARTN e25031 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruschwitz JD, List D, Waller L, Rubinov M, Walter H. GraphVar: A user-friendly toolbox for comprehensive graph analyses of functional brain connectivity. J Neurosci Meth. 2015;245:107–15. 10.1016/j.jneumeth.2015.02.021 [DOI] [PubMed] [Google Scholar]
- 17.Hosseini SMH, Hoeft F, Kesler SR. GAT: A Graph-Theoretical Analysis Toolbox for Analyzing Between-Group Differences in Large-Scale Structural and Functional Brain Networks. Plos One. 2012;7(7). ARTN e40709 10.1371/journal.pone.0040709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong GL, Rosa P, Carbonell F, Chen ZJ, He Y, Evans AC. Age- and Gender-Related Differences in the Cortical Anatomical Network. J Neurosci. 2009;29(50):15684–93. 10.1523/JNEUROSCI.2308-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s Disease. J Neurosci. 2008;28(18):4756–66. 10.1523/JNEUROSCI.0141-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, et al. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain. 2009;132:3366–79. 10.1093/brain/awp089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao W, Zhang ZQ, Pan ZY, Mantini D, Ding JR, Duan XJ, et al. Altered Functional Connectivity and Small-World in Mesial Temporal Lobe Epilepsy. Plos One. 2010;5(1). ARTN e8525 10.1371/journal.pone.0008525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Liang M, Zhou Y, He Y, Hao YH, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–61. 10.1093/brain/awn018 [DOI] [PubMed] [Google Scholar]
- 23.Gong GL, He Y, Concha L, Lebel C, Gross DW, Evans AC, et al. Mapping Anatomical Connectivity Patterns of Human Cerebral Cortex Using In Vivo Diffusion Tensor Imaging Tractography. Cerebral Cortex. 2009;19(3):524–36. 10.1093/cercor/bhn102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YH, Liu Y, Li J, Qin W, Li KC, Yu CS, et al. Brain Anatomical Network and Intelligence. Plos Computational Biology. 2009;5(5). ARTN e1000395 10.1371/journal.pcbi.1000395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Heuvel MP, Stam CJ, Kahn RS, Pol HEH. Efficiency of Functional Brain Networks and Intellectual Performance. J Neurosci. 2009;29(23):7619–24. 10.1523/JNEUROSCI.1443-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian F, Chen QL, Zhu WF, Wang YM, Yang WJ, Zhu XX, et al. The association between visual creativity and cortical thickness in healthy adults. Neurosci Lett. 2018;683:104–10. 10.1016/j.neulet.2018.06.036 [DOI] [PubMed] [Google Scholar]
- 27.Pinho AL, de Manzano O, Fransson P, Eriksson H, Ullen F. Connecting to Create: Expertise in Musical Improvisation Is Associated with Increased Functional Connectivity between Premotor and Prefrontal Areas. J Neurosci. 2014;34(18):6156–63. 10.1523/JNEUROSCI.4769-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J, Yang H, Zhang XX, He H, Luo C, Yao DZ. The Brain Functional State of Music Creation: an fMRI Study of Composers. Scientific Reports. 2015;5 Artn 12277 10.1038/Srep12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkamo T. Music for the ageing brain: Cognitive, emotional, social, and neural benefits of musical leisure activities in stroke and dementia. Dementia-International Journal of Social Research and Practice. 2018;17(6):670–85. 10.1177/1471301217729237 [DOI] [PubMed] [Google Scholar]
- 30.Lin CS, Liu Y, Huang WY, Lu CF, Teng S, Ju TC, et al. Sculpting the Intrinsic Modular Organization of Spontaneous Brain Activity by Art. Plos One. 2013;8(6). ARTN e66761 10.1371/journal.pone.0066761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–22. 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- 32.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–48. 10.1006/nimg.2002.1087 [DOI] [PubMed] [Google Scholar]
- 33.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–9. 10.1016/j.tics.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 34.Burkhouse KL, Kujawa A, Hosseini B, Klumpp H, Fitzgerald KD, Langenecker SA, et al. Anterior cingulate activation to implicit threat before and after treatment for pediatric anxiety disorders. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2018;84:250–6. 10.1016/j.pnpbp.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshino A, Okamoto Y, Okada G, Takamura M, Ichikawa N, Shibasaki C, et al. Changes in resting-state brain networks after cognitive-behavioral therapy for chronic pain. Psychological Medicine. 2018;48(7):1148–56. 10.1017/S0033291717002598 [DOI] [PubMed] [Google Scholar]
- 36.Winocur G. The Hippocampus and Thalamus—Their Roles in Short-Term and Long-Term-Memory and the Effects of Interference. Behavioural Brain Research. 1985;16(2–3):135–52. 10.1016/0166-4328(85)90088-9 [DOI] [PubMed] [Google Scholar]
- 37.Albin RL, Young AB, Penney JB. The Functional-Anatomy of Basal Ganglia Disorders. Trends in Neurosciences. 1989;12(10):366–75. 10.1016/0166-2236(89)90074-X [DOI] [PubMed] [Google Scholar]
- 38.Chang WH, Kim YH, Yoo WK, Goo KH, Park CH, Kim ST, et al. rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restorative Neurology and Neuroscience. 2012;30(3):179–89. 10.3233/RNN-2012-110162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Postle BR, M D’Esposito. Dissociation of human caudate nucleus activity in spatial and nonspatial working memory: an event-related fMRI study. Cognitive Brain Res. 1999;8(2):107–15. 10.1016/S0926-6410(99)00010-5 [DOI] [PubMed] [Google Scholar]
- 40.Schwabe L, Tegenthoff M, Hoffken O, Wolf OT. Mineralocorticoid Receptor Blockade Prevents Stress-Induced Modulation of Multiple Memory Systems in the Human Brain. Biological Psychiatry. 2013;74(11):801–8. 10.1016/j.biopsych.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 41.Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J Neurosci. 2003;23(13):5945–52. 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mcdonald RJ, White NM. Parallel Information-Processing in the Water Maze—Evidence for Independent Memory-Systems Involving Dorsal Striatum and Hippocampus. Behavioral and Neural Biology. 1994;61(3):260–70. 10.1016/S0163-1047(05)80009-3 [DOI] [PubMed] [Google Scholar]
- 43.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiology of Learning and Memory. 2002;77(2):125–84. 10.1006/nlme.2001.4008 [DOI] [PubMed] [Google Scholar]
- 44.Bender AD, Filmer HL, Dux PE. Transcranial direct current stimulation of superior medial frontal cortex disrupts response selection during proactive response inhibition. Neuroimage. 2017;158:455–65. Epub 2016/10/30. 10.1016/j.neuroimage.2016.10.035 . [DOI] [PubMed] [Google Scholar]
- 45.Chen CY, Muggleton NG, Tzeng OJL, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. Neuroimage. 2009;44(2):537–45. 10.1016/j.neuroimage.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 46.Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. Journal of Cognitive Neuroscience. 2006;18(11):1843–9. 10.1162/jocn.2006.18.11.1843 [DOI] [PubMed] [Google Scholar]
- 47.Hannon EE, Trehub SE. Tuning in to musical rhythms: Infants learn more readily than adults. P Natl Acad Sci USA. 2005;102(35):12639–43. 10.1073/pnas.0504254102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steele CJ, Bailey JA, Zatorre RJ, Penhune VB. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(3):1282–90. Epub 2013/01/18. 10.1523/JNEUROSCI.3578-12.2013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaty RE, Benedek M, Silvia PJ, Schacter DL. Creative Cognition and Brain Network Dynamics. Trends in Cognitive Sciences. 2016;20(2):87–95. 10.1016/j.tics.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beaty RE, Kenett YN, Christensen AP, Rosenberg MD, Benedek M, Chen QL, et al. Robust prediction of individual creative ability from brain functional connectivity. P Natl Acad Sci USA. 2018;115(5):1087–92. 10.1073/pnas.1713532115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. Neuroimage. 2012;59(2):1783–94. 10.1016/j.neuroimage.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 52.Min BK. A thalamic reticular networking model of consciousness. Theoretical Biology and Medical Modelling. 2010;7 Artn 10 10.1186/1742-4682-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Manzano O, Cervenka S, Karabanov A, Farde L, Ullen F. Thinking Outside a Less Intact Box: Thalamic Dopamine D2 Receptor Densities Are Negatively Related to Psychometric Creativity in Healthy Individuals. Plos One. 2010;5(5). ARTN e10670 10.1371/journal.pone.0010670 [DOI] [PMC free article] [PubMed] [Google Scholar]












 The words 書 shū, 'script' (top), and 道 dào, 'way' (bottom), according to the main five styles of Chinese calligraphy.
The words 書 shū, 'script' (top), and 道 dào, 'way' (bottom), according to the main five styles of Chinese calligraphy.
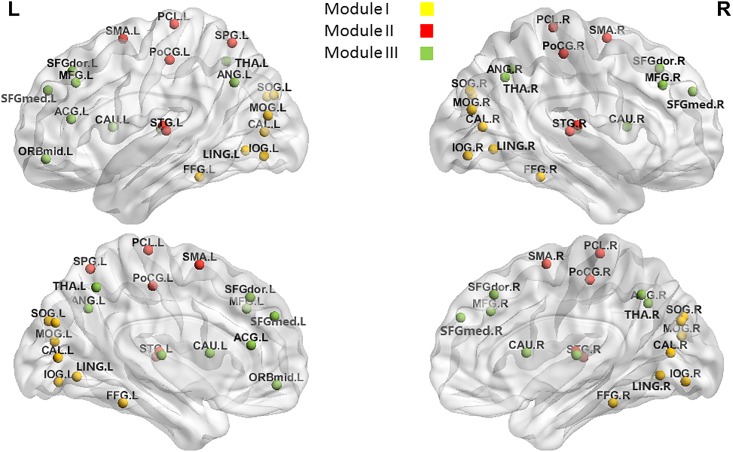
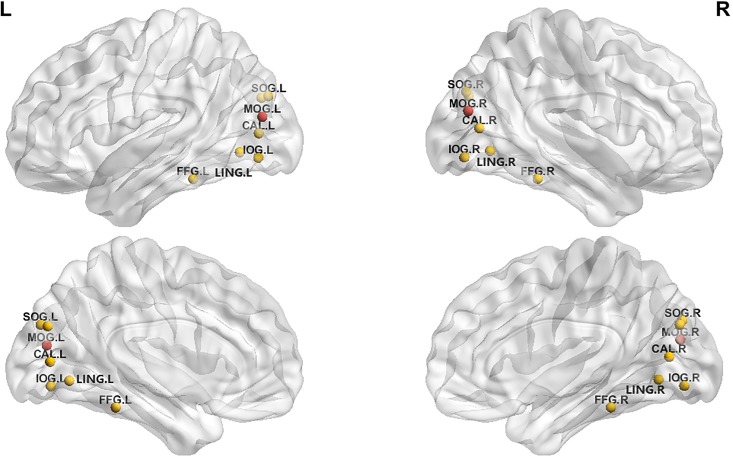
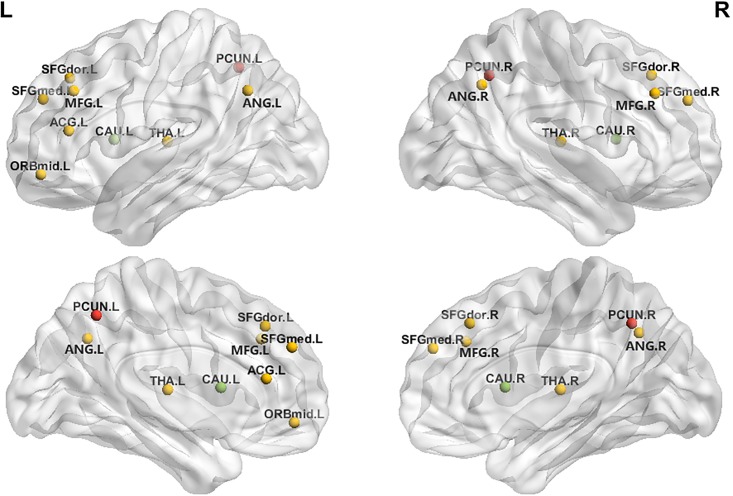

No comments:
Post a Comment